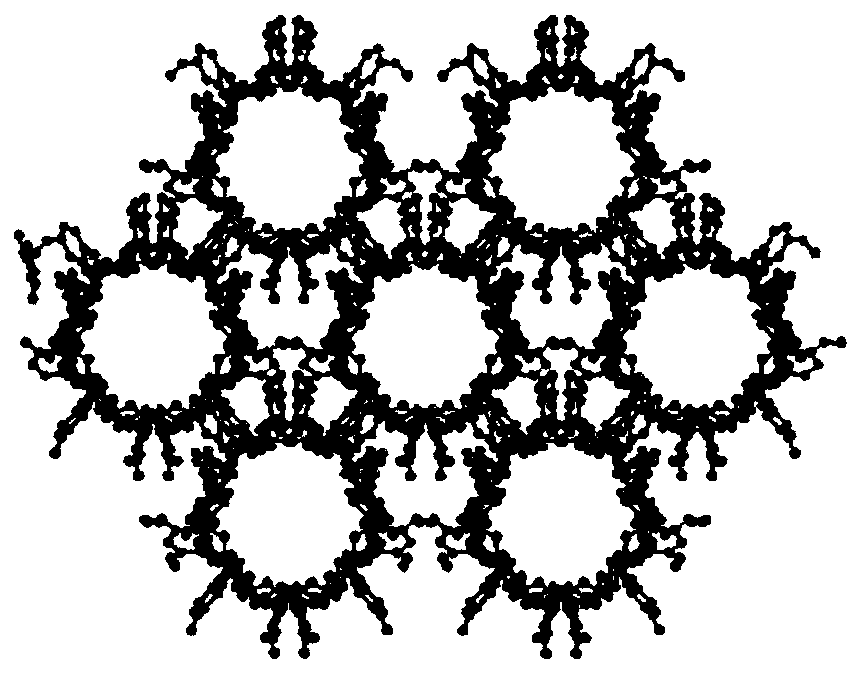
Preparation method of cyclodextrin constructed adsorbing material based on micropore structure
A technology of adsorption materials and microporous structures, applied in chemical instruments and methods, alkali metal oxides/hydroxides, inorganic chemistry, etc., to achieve the effects of easy mastery, simple methods, and simple operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
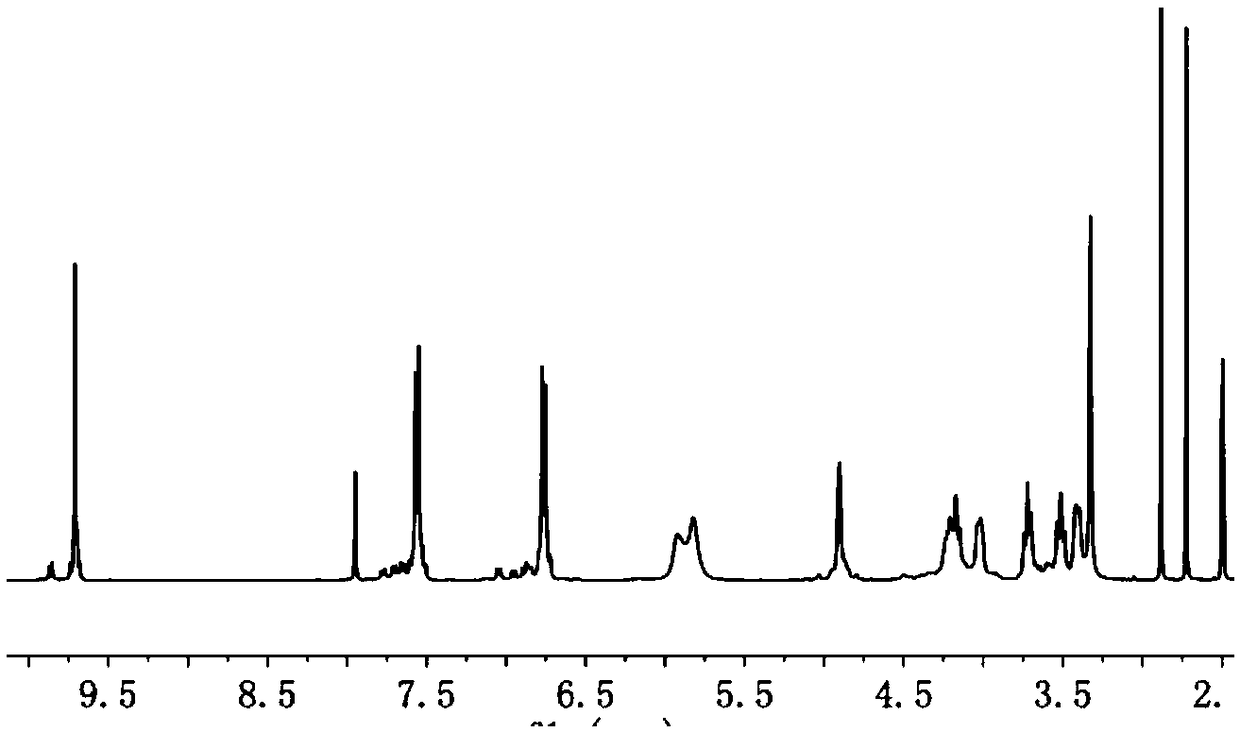
Image
Examples
Embodiment 1
[0023] A method for preparing an adsorption material based on microporous cyclodextrin, comprising the steps of:
[0024] (1) Preparation of Vilsmeier-Haack reagent.
[0025] Under anaerobic and anhydrous conditions, 14g triphenylphosphine was dissolved in N,N-dimethylformamide (DMF), then 8.6g bromine was added dropwise, and after 0.5h reaction, the Vilsmeier-Haack reagent ([ (CH3)2NCHBr]Br);
[0026] (2) Preparation of perbromo-β-cyclodextrin.
[0027] The DMF solution of β-cyclodextrin (3.4g) was added to the Vilsmeier-Haack reagent prepared in step (1), and the reaction temperature was controlled below 60°C for 16 hours. After the reaction was completed, the pH of the solution was adjusted to 8 with sodium methoxide, and then Add methanol to precipitate, and filter with suction to obtain perbromo-β-cyclodextrin;
[0028] (3) Preparation of all-[6-oxo-6-(4-formylbenzene)]-β-cyclodextrin adsorption material.
[0029] Dissolve 1.22g of p-hydroxybenzaldehyde in DMF, add 1....
Embodiment 2
[0033] A method for preparing an adsorption material based on microporous cyclodextrin, comprising the steps of:
[0034] (1) Preparation of Vilsmeier-Haack reagent.
[0035] Under anaerobic and anhydrous conditions, 14g triphenylphosphine was dissolved in N,N-dimethylformamide (DMF), then 8.6g bromine was added dropwise, and after 0.5h reaction, the Vilsmeier-Haack reagent ([ (CH3)2NCHBr]Br);
[0036] (2) Preparation of perbromo-α-cyclodextrin.
[0037] The DMF solution of α-cyclodextrin (2.9g) was added to the Vilsmeier-Haack reagent prepared in step (1), and the reaction temperature was controlled below 60°C for 8 hours. After the reaction was completed, the pH of the solution was adjusted to 8 with sodium methoxide, and then Add methanol to precipitate, and filter with suction to obtain perbromo-α-cyclodextrin;
[0038] (3) Preparation of all-[6-oxo-6-(4-formylbenzene)]-α-cyclodextrin adsorption material.
[0039] Dissolve 1.22g of p-hydroxybenzaldehyde in DMF, add 1.3...
Embodiment 3
[0043] A method for preparing an adsorption material based on microporous cyclodextrin, comprising the steps of:
[0044] (1) Preparation of Vilsmeier-Haack reagent.
[0045] Under anaerobic and anhydrous conditions, 14g triphenylphosphine was dissolved in N,N-dimethylformamide (DMF), then 8.6g bromine was added dropwise, and after 0.5h reaction, the Vilsmeier-Haack reagent ([ (CH3)2NCHBr]Br);
[0046] (2) Preparation of perbromo-γ-cyclodextrin.
[0047] The DMF solution of γ-cyclodextrin (3.9g) was added to the Vilsmeier-Haack reagent prepared in step (1), and the reaction temperature was controlled below 60°C for 16 hours. After the reaction was completed, the pH of the solution was adjusted to 7 with sodium methoxide, and then Add methanol to precipitate, and filter with suction to obtain perbromo-γ-cyclodextrin;
[0048] (3) Preparation of all-[6-oxo-6-(4-formylbenzene)]-γ-cyclodextrin adsorption material.
[0049] Dissolve 1.22g of p-hydroxybenzaldehyde in DMF, add 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com