NNN tridentate ligand with far-end hydroxyl group, preparation method and application thereof, and ruthenium complex
A technology of ruthenium complexes and hydroxyl groups is applied to ruthenium complexes, NNN tridentate ligands and their preparation methods and application fields, which can solve the problems of modulation limitation and the like, and achieve the effect of excellent catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The present invention also provides a preparation method of the NNN tridentate ligand having a remote hydroxyl group described in the above technical solution. When the NNN tridentate ligand having a distal hydroxyl group has a structure shown in formula A or B, the preparation method includes the following steps:
[0039] Under the protection of nitrogen, after mixing 3,5-dimethyl-1H-pyrazole and DMF, NaH was added in batches in an ice-water bath, and then 2,6-difluoropyridine was added dropwise for monoamination reaction to obtain 2-(3,5-Dimethyl-1H-pyrazol-1-yl)-6-fluoropyridine;
[0040] Under nitrogen protection, after mixing the compound with the structure shown in formula I and DMF, in an ice-water bath, add NaH in batches, and then combine with the 2-(3,5-dimethyl-1H-pyrazole- 1-yl)-6-fluoropyridine is mixed for amination reaction to obtain a compound having the structure shown in formula II, and the compound having the structure shown in formula I is ethyl 1H-...
Embodiment 1
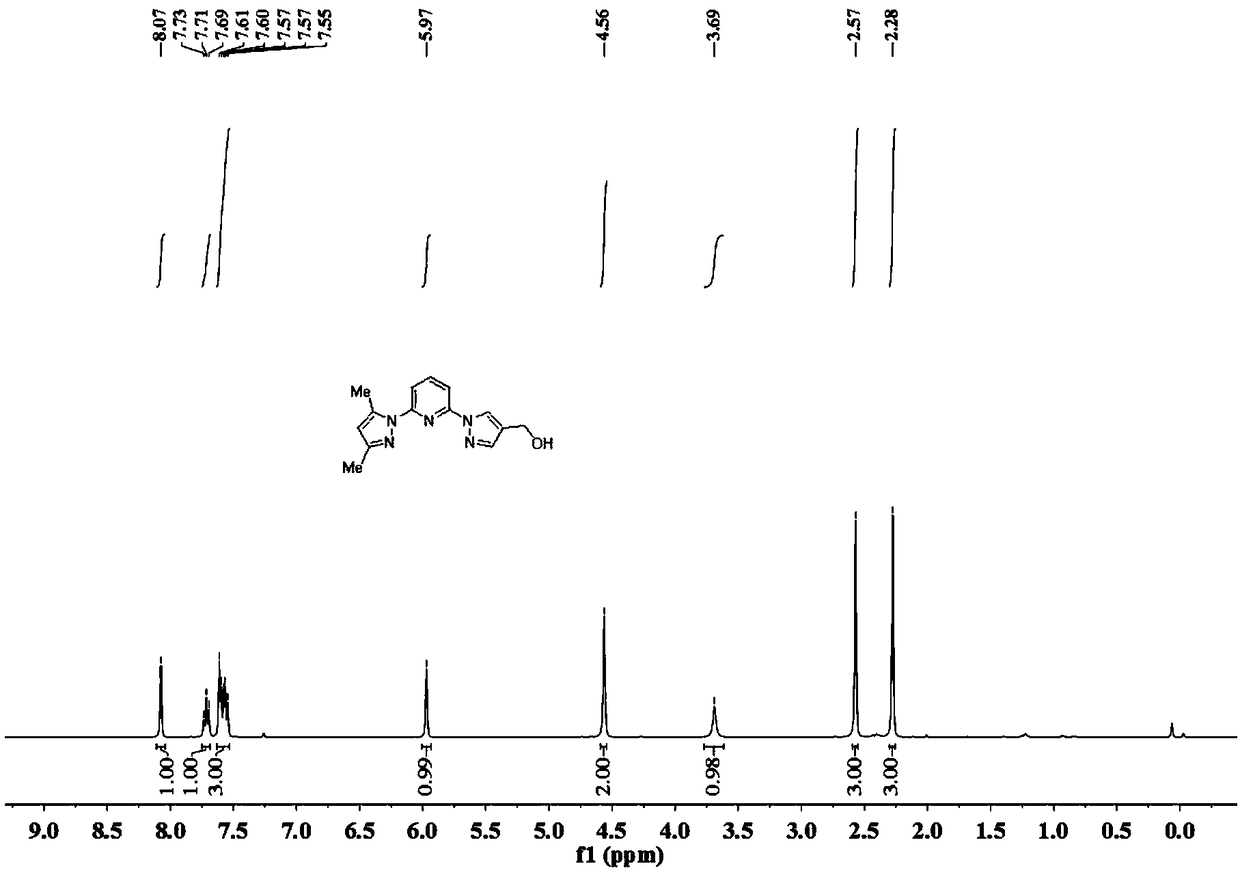
[0099] Preparation of Ligand A
[0100] Step 1: under nitrogen protection, 3,5-dimethyl-1H-pyrazole (16.7g, 1.74mol) was dissolved in dry DMF (60mL), and 60% NaH was added in four equal batches at 0°C ( 9.0 g, 2.26 mol). After stirring for 30 min, 2,6-difluoropyridine was added dropwise, warmed to room temperature and stirred for 4 h. Add an appropriate amount of water to quench the reaction, extract three times with dichloromethane, combine the organic phases, and wash with MgSO 4 dry. Filter out MgSO 4 , the solvent was removed, and the crude product was subjected to column chromatography to obtain a white solid (24 g, yield 73%).
[0101] Step 2: Under nitrogen protection, 1H-pyrazole-4-carboxylic acid ethyl ester (5.6g, 40.3mmol) was dissolved in dry DMF (30mL), and 60% NaH (1.6g , 40.3 mmol). After stirring for 15 min, 2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-fluoropyridine (6.0 g, 31 mmol) was added in batches, and the temperature was raised to 110° C. and stirred overni...
Embodiment 2
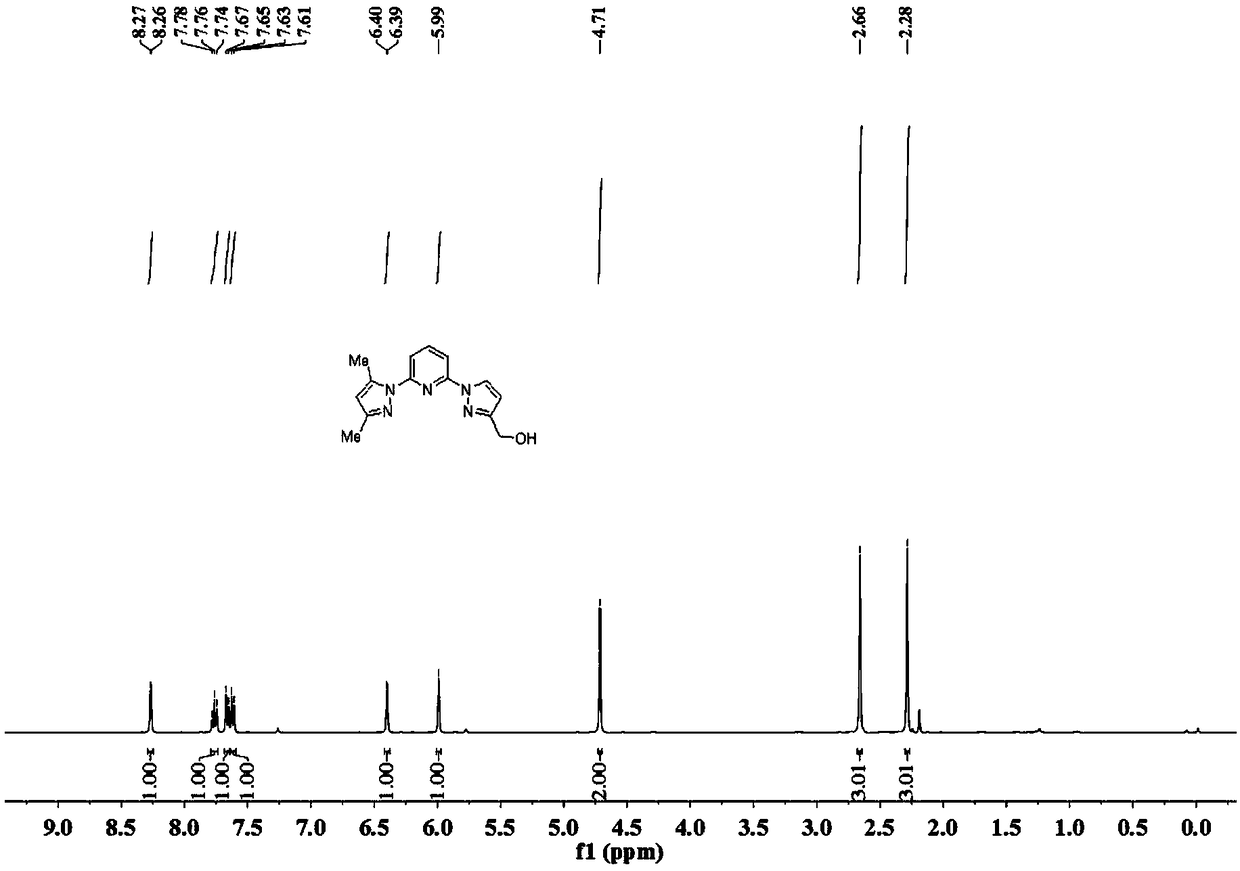
[0105] Preparation of Ligand B
[0106] Step 1: under nitrogen protection, 3,5-dimethyl-1H-pyrazole (16.7g, 1.74mol) was dissolved in dry DMF (60mL), and 60% NaH (9.0 g, 2.26mol). After stirring for 30 min, 2,6-difluoropyridine was added dropwise, warmed up to room temperature and stirred for 4 h. Add an appropriate amount of water to quench the reaction, extract three times with dichloromethane, combine the organic phases, MgSO 4 Dry for 2h. Filter out MgSO 4 , the solvent was evaporated by rotary evaporation, and the crude product was subjected to column chromatography to obtain a white solid (24 g, yield 73%).
[0107] Step 2: Under nitrogen protection, 1H-pyrazole-3-carboxylic acid ethyl ester (3.7g, 26.2mmol) was dissolved in dry DMF (25mL), and 60% NaH (1.0g , 26.2 mmol). After stirring for 15 min, 2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-fluoropyridine (4.0 g, 20.9 mmol) was slowly added, and the temperature was raised to 110° C. and stirred overnight. Add an appropria...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com