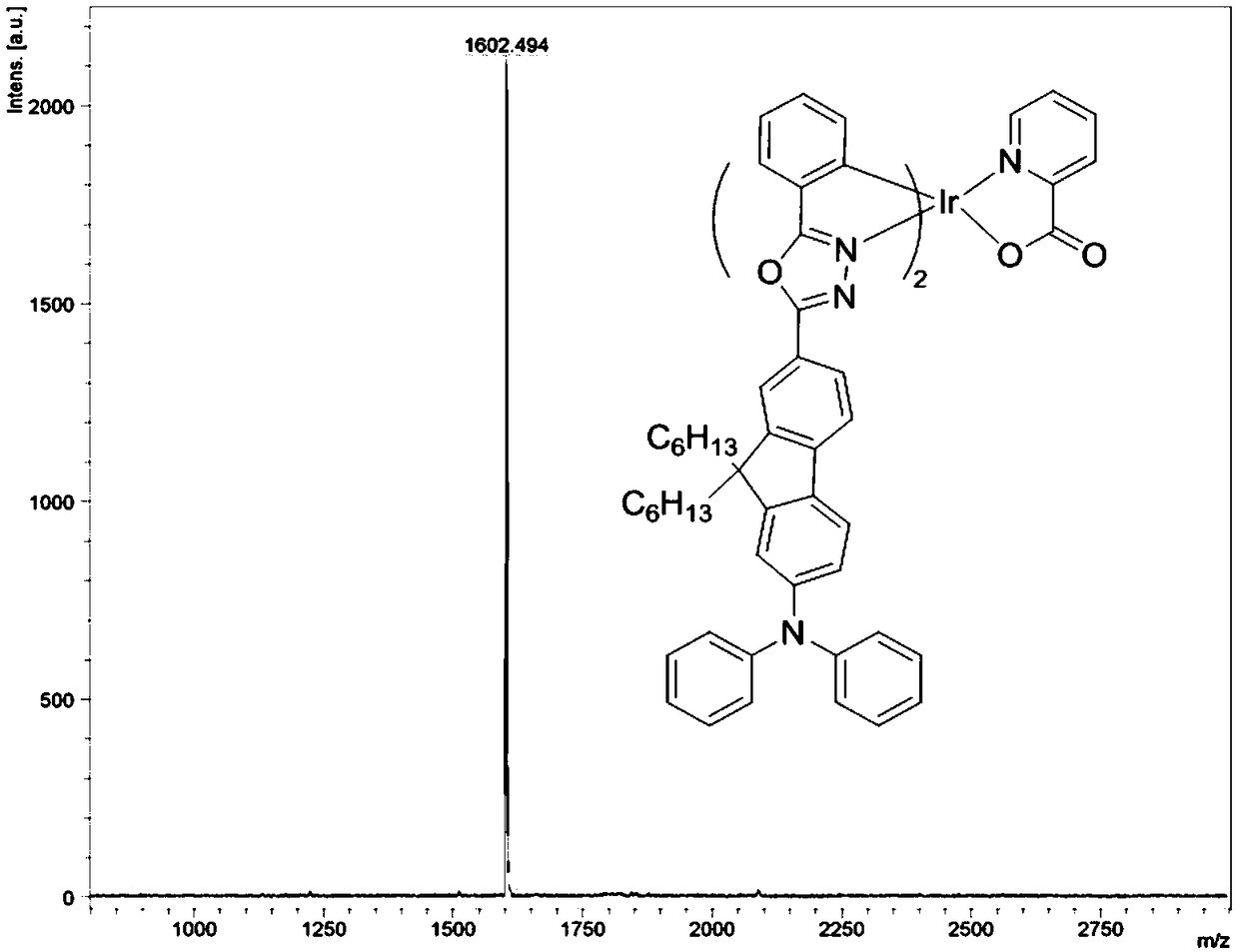
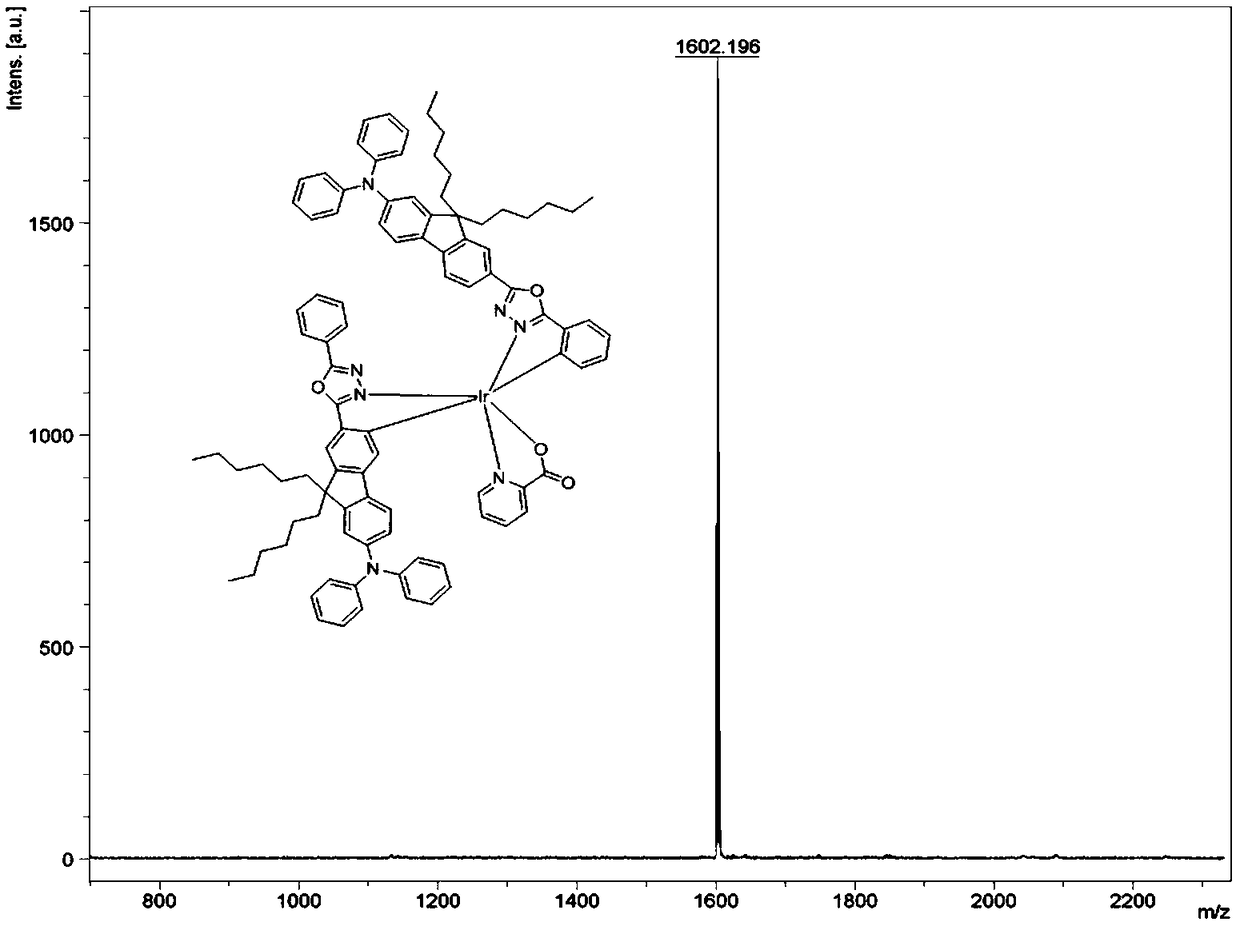
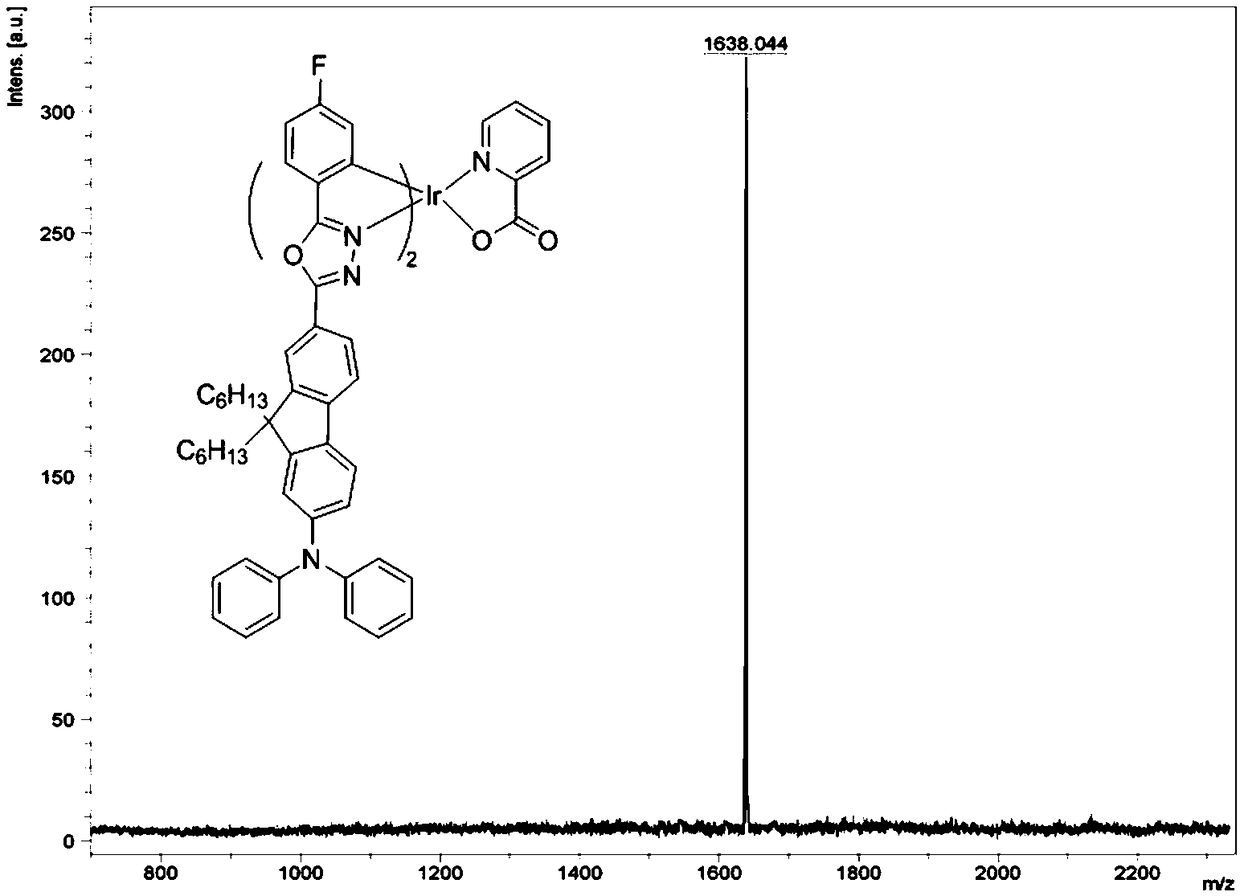
Bipolar phosphorescent iridium complex based on fluorenyl-oxadiazole, and preparation method and applications thereof
A technology of phosphorescent iridium complexes and oxadiazoles, which is applied in the field of organic phosphorescent materials, can solve the problems of oxadiazoles that are rarely reported, and achieve the effects of simple preparation, improved luminous efficiency, and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Synthesis and characterization of 2-bromo-7-iodo-9,9-dihexylfluorene.
[0046] Weigh 2-bromo-7-iodo-fluorene (25.641g, 68.01mmol), a mixture of bromo-n-hexane (33.664g, 204.02mmol) and sodium hydroxide (11.002g, 275.1mmol) was dissolved in DMSO, at 55 After reacting at constant temperature for 10 hours at ℃, the DMSO solvent was removed by repeated extraction with water and ethyl acetate, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, and use petroleum ether as an eluent for column chromatography to obtain a white product; dry, weigh, and calculate to obtain a product yield of 90%.
[0047]
[0048] (2) Synthesis and characterization of 2-N,N-diphenyl-7-bromo-9,9-dihexyl-fluorene.
[0049] Weigh 2-bromo-7-iodo-9,9-dihexylfluorene (7.64g, 14.12mmol), diphenylamine (2.213g, 12.82mmol), CuI (0.139g, 0.715) and potassium tert-butoxide (t -BuOK) (4.853g, 42.38mmol) was put into a three-necked flask, and after the nitrogen was replaced three times, 1...
Embodiment 2
[0069] (1) Synthesis of 2-bromo-7-iodo-9,9-dihexylfluorene.
[0070] Weigh 2-bromo-7-iodo-fluorene (25.641g, 68.01mmol), a mixture of bromo-n-hexane (33.664g, 204.02mmol) and sodium hydroxide (11.002g, 275.1mmol) was dissolved in DMSO, at 55 After reacting at constant temperature for 10 hours, extract with water and ethyl acetate to remove the DMSO solvent, dry the organic phase with anhydrous Na2SO4, and use petroleum ether as the eluent for column chromatography to obtain a white product; dry and weigh , The calculated product yield is 90%.
[0071] (2) Synthesis of 2-N,N-diphenyl-7-bromo-9,9-dihexyl-fluorene.
[0072] Weighed 2-bromo-7-iodo-9,9-dihexylfluorene (7.64g, 14.12mmol), diphenylamine (2.213g, 12.82mmol), CuI (0.139g, 0.715) and t-BuOK (4.853g , 42.38mmol) into a three-necked flask, and after replacing nitrogen three times, 15ml of toluene was added to these mixtures as a reaction solvent, and reacted at 110° C. for 8 hours. The reaction was extracted with dichl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com