D-A type organic light-emitting material and preparation method and application thereof
A luminescent material, D-A technology, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of lack of high efficiency and stability, high cost of phosphorescent emission materials, etc., achieve simple process, concise and ingenious synthesis route, and improve efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
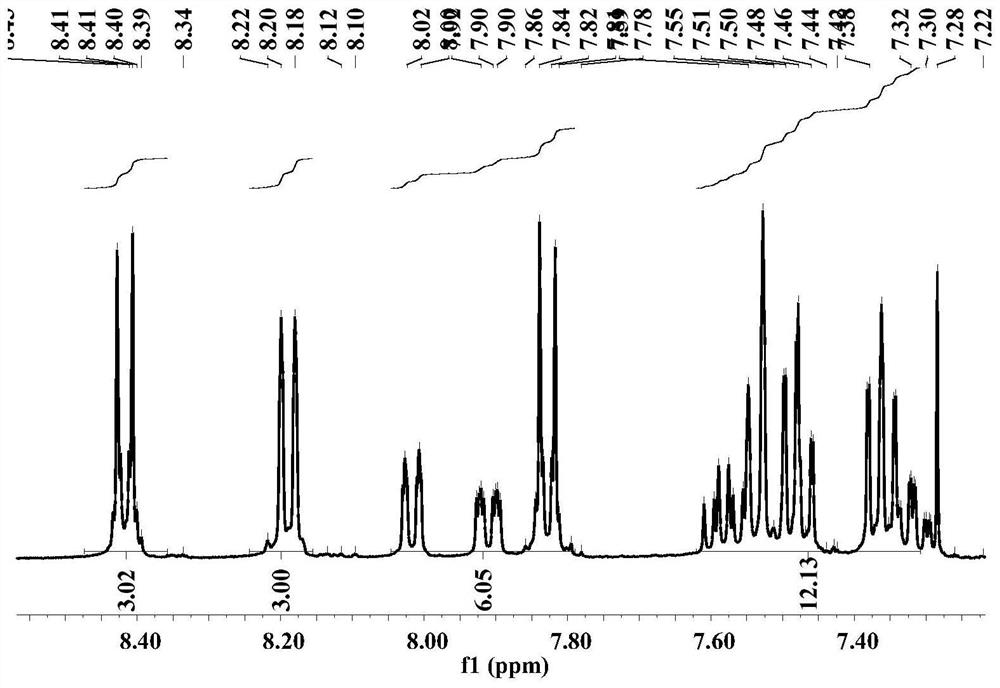
Embodiment 1
[0060] The preparation method of compound d-CzOXD-3 comprises the following steps:
[0061] (1) Synthesis of compound 1a:
[0062]
[0063] Weigh 9-(4-bromophenyl)carbazole (20g, 62.11mmol), CuCN (11.17g, 124.22mmol) was added to the three-necked flask equipped with the condensation device, after 3 times of evacuation and nitrogen filling, 25mL was added with a syringe After the reaction was cooled to room temperature, 20 mL of water was added, then 10 mL of ammonia water was added while stirring, stirred for 30 min, suction filtration, and the solid obtained by suction filtration was extracted with dichloromethane, and the waterNa 2 SO 4 After drying, the crude product was purified by column chromatography (eluent: dichloromethane:petroleum ether=1:4) to obtain 12 g of white solid, yield: 72%.
[0064] (2) Synthesis of compound 2a:
[0065]
[0066] Compound 1a (12g, 44.78mmol), NaN was added to a 250mL three-necked reaction flask 3 (5.82 g, 89.56 mmol) and triethy...
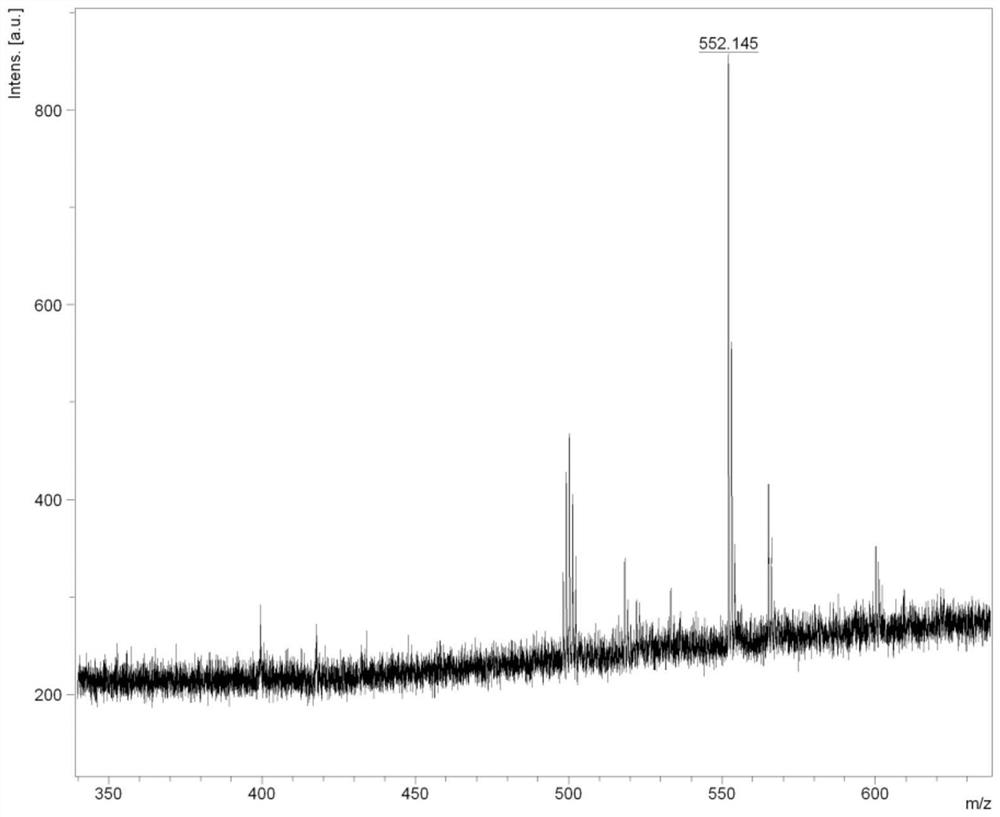
Embodiment 2
[0074] The preparation method of compound d-CzOXD-24 comprises the following steps:
[0075] Steps (1) and (2) are the same as in Example 1.
[0076] (3) Synthesis of compound 3b:
[0077]
[0078] Under nitrogen protection, compound 2a (1.0 g, 3.21 mmol) and 2,4-difluorobenzoyl chloride (1.133 g, 6.42 mmol) were added to a 100 mL three-necked reaction flask, 15 mL of pyridine was added, and the reaction was carried out at 110 °C for 24 h in an oil bath. . After cooling to room temperature, add excess dilute hydrochloric acid solution to remove unreacted pyridine, continue stirring for 6h, and then extract with ethyl acetate and deionized water for several times. The upper organic phase is dried with anhydrous sodium sulfate and dried with a rotary evaporator. Ethyl acetate, the crude product was purified by column chromatography (eluent: dichloromethane: petroleum ether=3:1) to obtain 1.2 g of white solid, yield: 85%.
[0079] (4) Synthesis of compound d-CzOXD-24:
[0...
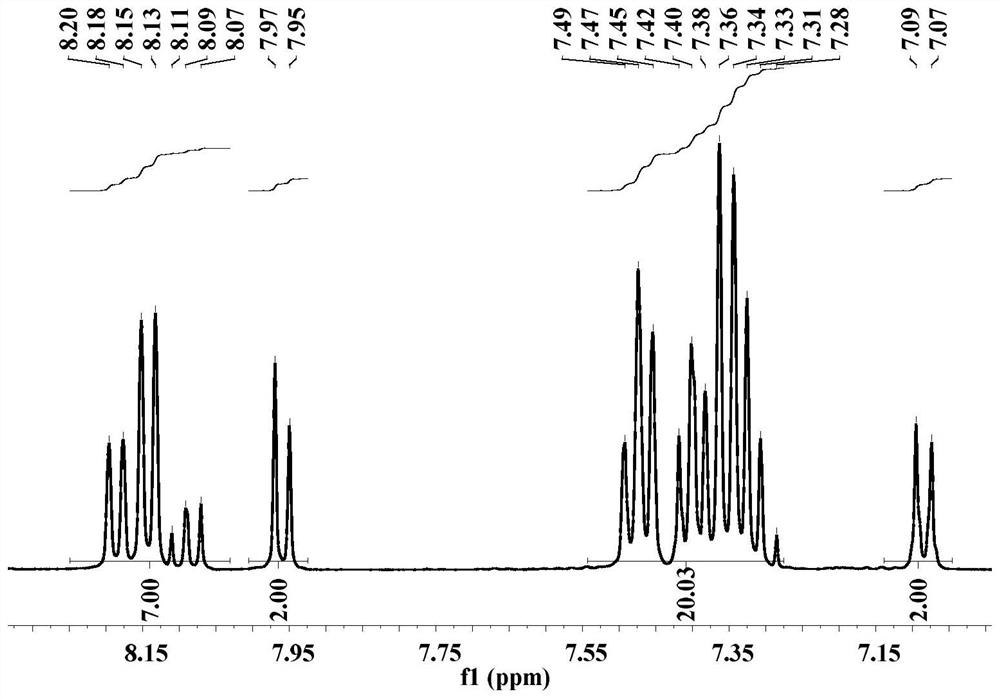
Embodiment 3
[0083] The preparation method of compound d-CzOXD-26 comprises the following steps:
[0084] Steps (1) and (2) are the same as in Example 1.
[0085] (3) Synthesis of compound 3c:
[0086]
[0087] Under nitrogen protection, compound 2a (1.0 g, 3.21 mmol) and 2,6-difluorobenzoyl chloride (1.133 g, 6.42 mmol) were added to a 100 mL three-necked reaction flask, 15 mL of pyridine was added, and the reaction was carried out at 110 °C in an oil bath for 24 h. After cooling to room temperature, add excess dilute hydrochloric acid solution to remove unreacted pyridine, continue stirring for 6h, and then extract with ethyl acetate and deionized water for several times. The upper organic phase is dried with anhydrous sodium sulfate and dried with a rotary evaporator. Ethyl acetate, the crude product was purified by column chromatography (eluent: dichloromethane:petroleum ether=3:1) to obtain 1.18 g of white solid, yield: 85%.
[0088] (4) Synthesis of compound d-CzOXD-26:
[0089...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com