Anthracene derivative and preparation method and application thereof
A technology of anthracene derivatives and compounds, applied in the field of anthracene derivatives and their preparation, can solve the problem of low fluorescence quantum yield, achieve the effects of improving fluorescence quantum yield, inhibiting π-π stacking, and reducing injection barriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
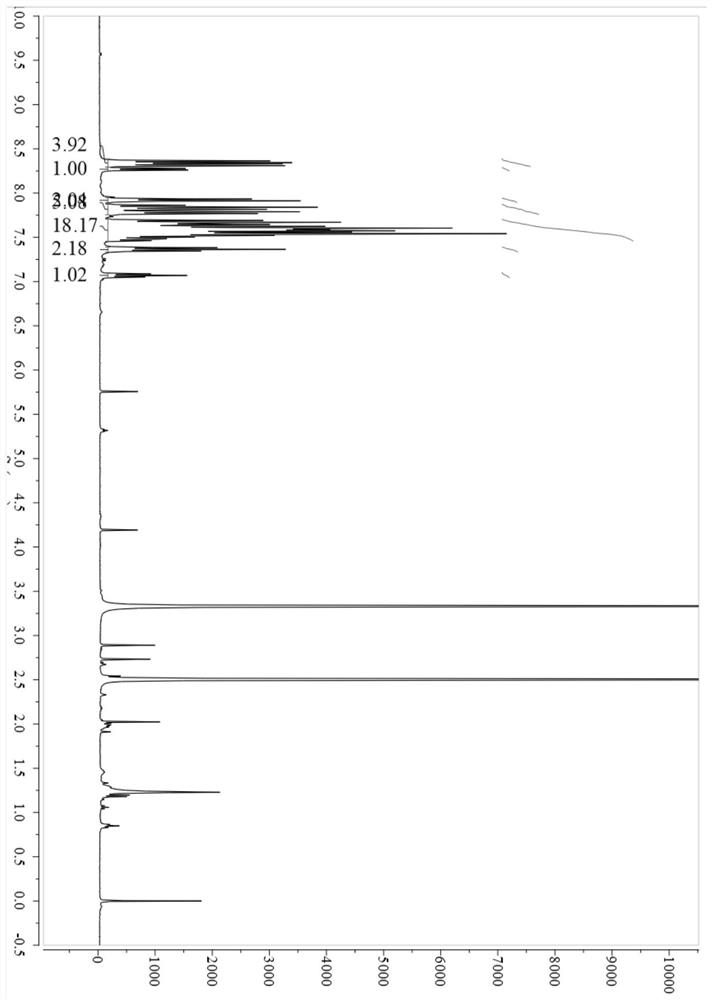
[0062] An anthracene derivative with the molecular structure described in the following formula, named pipdAnCz:
[0063]
[0064] The preparation method of the anthracene derivative comprises the following steps:
[0065] Preparation of S1.9-(4-(10-boryl anthracene-9-yl)phenyl)-9H-carbazole:
[0066] 9-(4-(10-bromoanthracen-9-yl)phenyl)-9H-carbazole (1g, 2mmol), pinacol diboronate (0.76g, 3mmol), potassium acetate (0.4g, 4mmol ), bistriphenylphosphine palladium dichloride (0.07g, 0.1mmol) were successively added in a 100ml two-necked flask, the flask was evacuated under vacuum and replaced three times in dry nitrogen, and then added 1,4-dioxane alkane (30mL), stirred and reacted at 100°C for 24h, extracted with saturated brine and dichloromethane, and distilled under reduced pressure to obtain a black solid, using silica gel powder as the stationary phase, petroleum ether / dichloromethane as the eluent, Obtain 0.77g of white powder (yield 71%) by column chromatography;
...
Embodiment 2
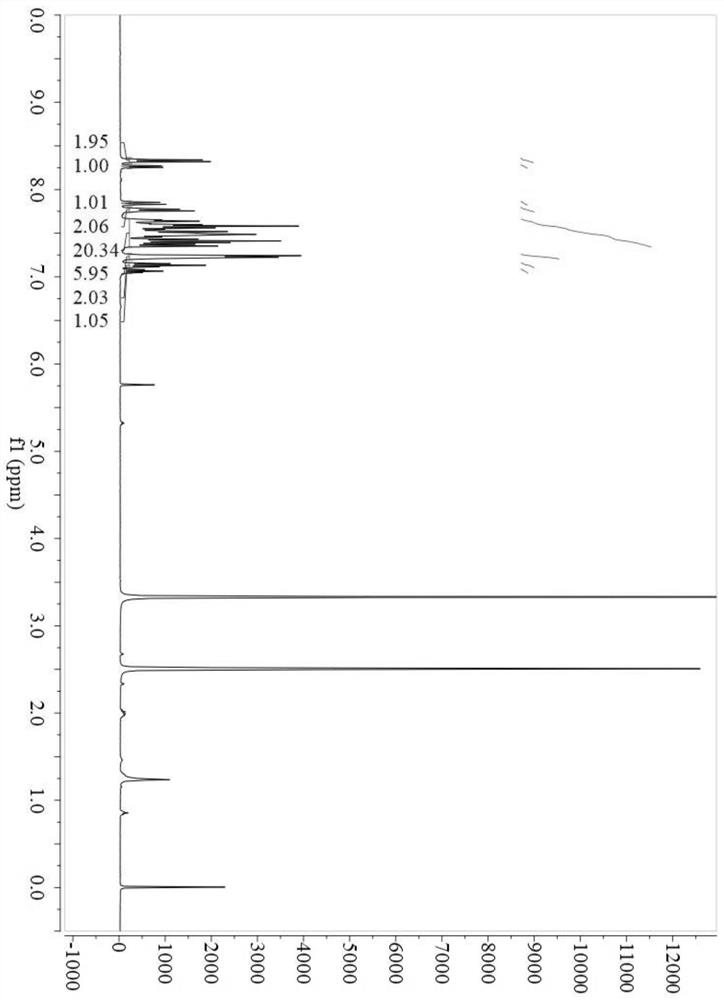
[0074] A kind of anthracene derivative, has the molecular structure described in the following formula, named pipdAnTPA:
[0075]
[0076] The preparation method of the anthracene derivative comprises the following steps:
[0077] Preparation of S1.4-(10-boryl anthracene-9-yl)-N,N-diphenylaniline:
[0078] 4-(10-bromoanthracen-9-yl)-N,N-diphenylaniline (1g, 2mmol), pinacol diboronate (0.76g, 3mmol), potassium acetate (0.4g, 4mmol), Bistriphenylphosphine palladium dichloride (0.07g, 0.1mmol) was successively added in a 100ml two-necked flask, the flask was evacuated under vacuum and replaced three times in dry nitrogen, then added 1,4-dioxane ( 30mL), stirred and reacted at 100°C for 24h, extracted with saturated saline and dichloromethane, and distilled under reduced pressure to obtain a black solid, using silica gel powder as the stationary phase and petroleum ether / dichloromethane as the eluent, passing through the column Chromatography obtained 0.84 g of yellow powder ...
Embodiment 3
[0086] This embodiment provides an anthracene derivative, the preparation method of which is similar to that of Example 1. The molecular structural formula of the anthracene derivative is as follows:
[0087]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com