Sheep pox inactivated vaccine and preparation method thereof
An inactivated vaccine and sheeppox technology, applied in the field of inactivated sheeppox vaccine and its production, can solve the problems of needing intradermal immunization and difficult operation of live sheeppox vaccine, and achieve the effect of avoiding biosafety risks.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
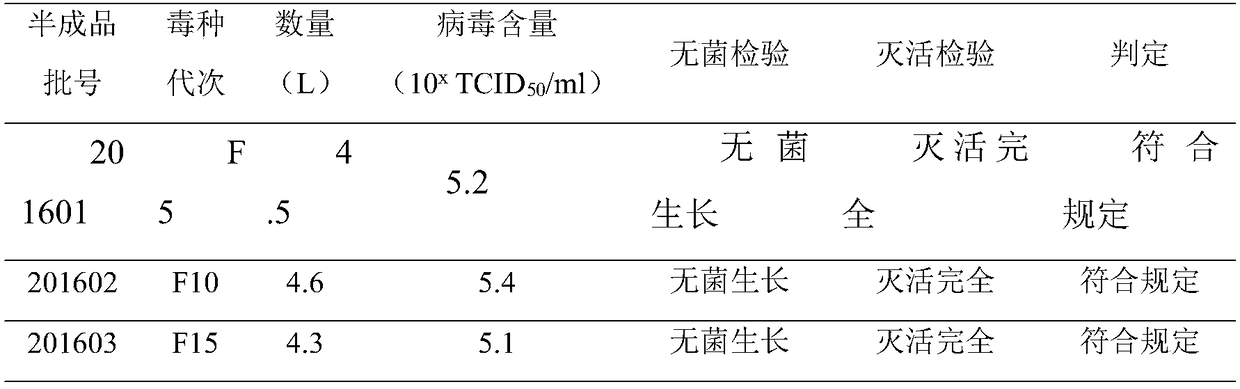
[0037] —— Vaccine preparation and testing
[0038] According to the established production process, three batches of inactivated sheeppox vaccine were prepared, and the results are reported as follows.
[0039] 1. Materials
[0040] (1) The cells used for cell production are BHK-21 suspension cell strains, which are preserved by the Institute (see China Veterinary Drug Administration, China Veterinary Microbial Cultures Preservation Management Center, Chinese Veterinary Strain Catalog, Chinese Agricultural Science and Technology Press, 2008, p147).
[0041] (2) Virus sheep pox virus AV41 strain and AV40 strain.
[0042] (3) Oil adjuvant Montanide ISA 201 adjuvant, produced by SEPPIC, France.
[0043] (4) Experimental animals: 3-month-old healthy and susceptible Inner Mongolia cashmere goats, free from infectious diseases, skin diseases and parasitic infections. Blood was collected before immunization to determine the neutralizing antibody titer of goat pox virus, and sheep...
Embodiment 2
[0073] ——Comparative research report with similar products
[0074] In order to compare the differences in safety, efficacy and immune production period between the inactivated sheeppox vaccine (AV41 strain, suspension culture) and similar products, three batches of trial-produced sheeppox inactivated vaccine (AV41 strain, suspension culture) products and domestic Similar products, compare the safety and efficacy of two vaccinations (21-day challenge protection rate after immunization).
[0075] 1 Materials and methods
[0076] 1.1 Materials
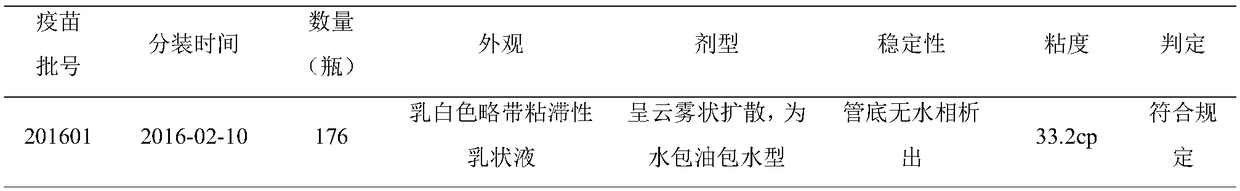
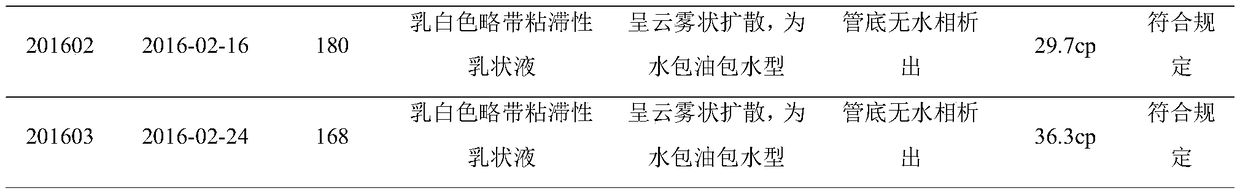
[0077] 1.1.1 Experimental vaccines Three batches of goat pox inactivated vaccines: batch numbers 201601, 201602 and 201603 (30 bottles each), prepared by our laboratory.
[0078] 1.1.2 Control vaccine Goat pox live vaccine, batch number 1605001, produced by China Animal Husbandry Co., Ltd. Lanzhou Biopharmaceutical Factory.
[0079] 1.1.3 AV40 strain, F3 generation, 1.0g / bottle, is identified, kept and supplied by China Veterinary Dru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com