Long-acting anticoagulant fusion protein and use thereof
A fusion protein and anticoagulant technology, applied in the direction of fusion polypeptide, protease inhibitor, peptide/protein components, etc., can solve the problems of thrombocytopenia, bleeding, short plasma half-life, etc., and achieve the effect of preventing and treating thrombosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: the preparation of fusion protein and in vitro anticoagulant activity assay
[0098] 1.1 Preparation of fusion protein
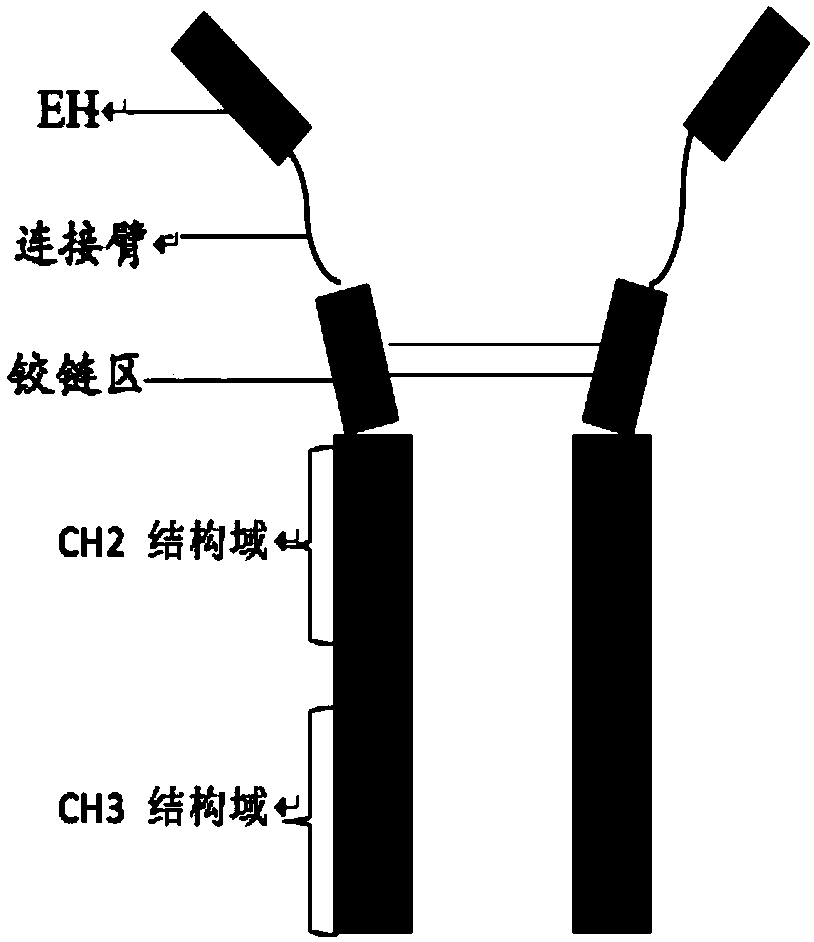
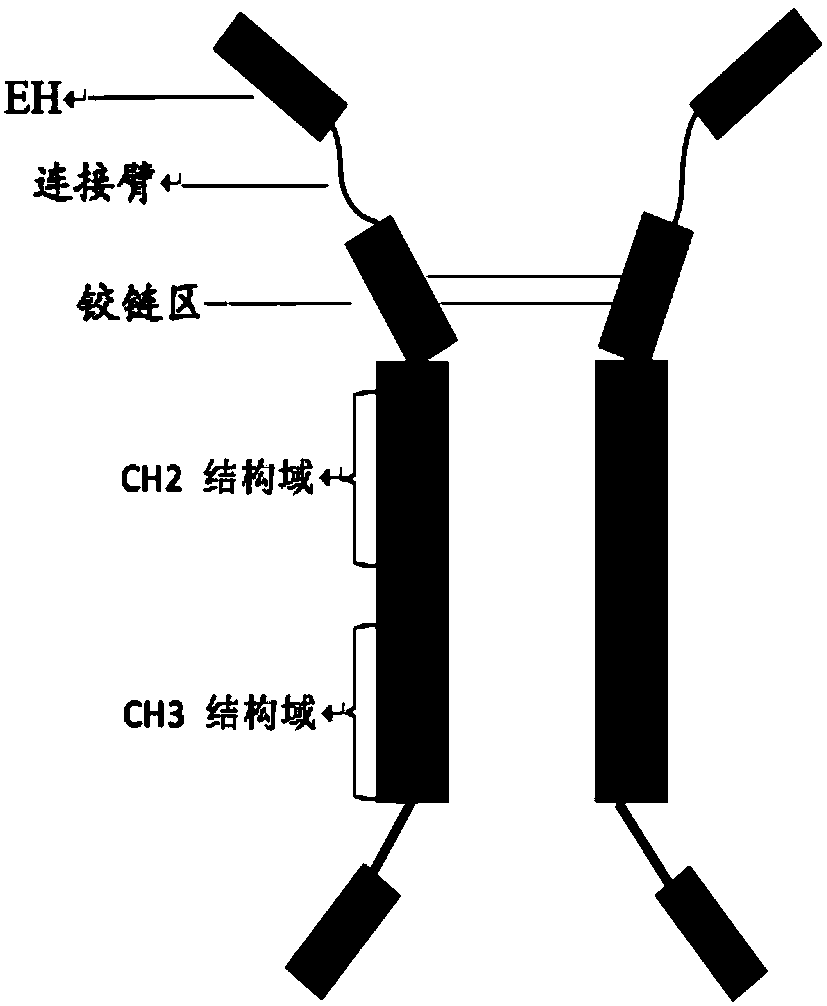
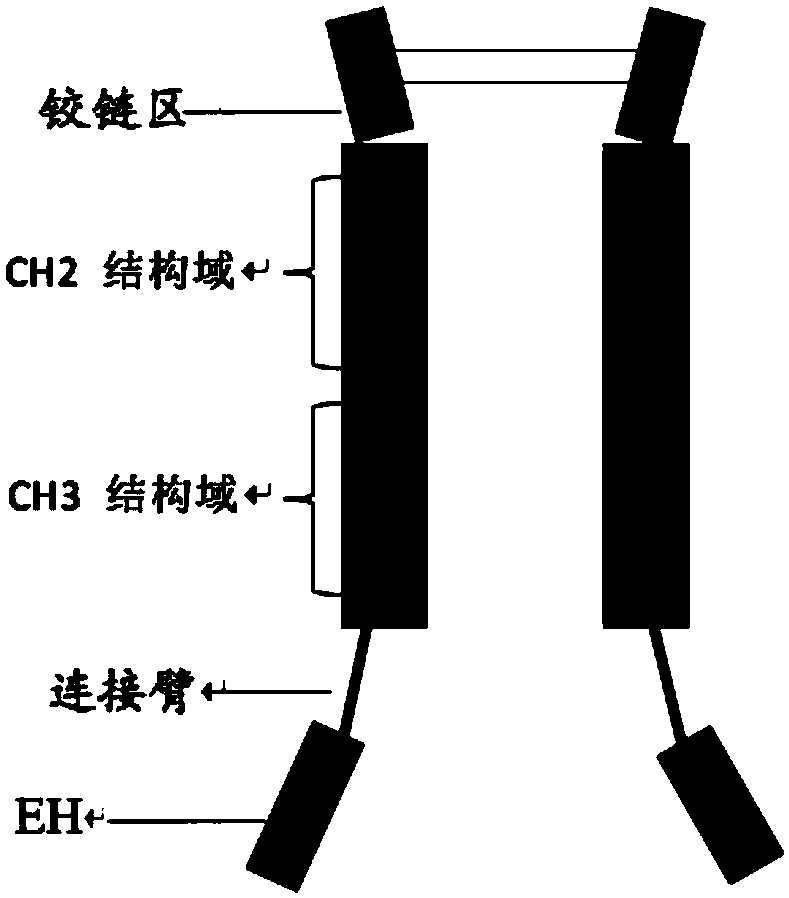
[0099] The nucleotide sequence encoding the fusion protein was constructed according to the construction method of each fusion protein shown in Table 2 by whole gene synthesis, wherein, the schematic diagram of the construct of EH-L-IgG1Fc, EH-L-IgG4Fc is as follows figure 1 As shown, the schematic diagram of the construct of EH-L-IgG4Fc-HV2 is shown in figure 2 As shown, the schematic diagram of the construct of IgG4Fc-L-EH is shown in image 3 As shown, the schematic diagram of the construct of IgG4Fc-EH is shown in Figure 4 shown. The nucleotide sequences encoding the above-mentioned fusion proteins were introduced into expression vectors by double enzyme digestion, and the obtained recombinant expression vectors were transfected into CHO cells by using liposomes, and the stably expressed expression vectors were obtained by pr...
Embodiment 2
[0124] Embodiment 2: the plasma half-life determination of fusion protein
[0125] 2.1 Experimental animals
[0126] Wistar rats, male, SPF grade, weighing 200-250g, were purchased from Beijing Weitong Lihua Company, and were raised in the experimental animal room of the Military Medical Research Institute. The experimental animals were given corresponding animal welfare and followed the 3R principles of animal experiments. start an experiment.
[0127] 2.2 Experimental method
[0128] The Wistar rats were randomly divided into 3 groups, 6 rats in each group, and IgG4Fc-EH (FcEH) was injected into the tail vein at low, medium and high doses (chemical doses were 4.5, 13.5, 27 mg / kg, respectively). Before administration and after administration 0.5h, 1h, 2h, 4h, 12h, 24h, 48h, 72h, 96h, 120h and 144h, blood was taken from the orbital venous plexus of rats, and 3.8% sodium citrate (Sinopharm Chemical Reagent Company) solution anticoagulant, separated plasma (3000r / min rota...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com