Rivaroxaban isopropylidene impurity reference substance and preparation method of reference substance
A technology of impurity reference substance, rivaroxaban, applied in the direction of organic chemistry, etc., to achieve the effect of simple process, simple preparation method and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of the rivaroxaban mesopropylidene impurity reference substance is as follows:
[0028] 1) Take 4.5g (15mmol) of 4-(4-(5-(aminomethyl)-2-oxo-3-oxazolidinyl)phenyl)-3-morpholinone, add 6.4g (60mmol) Sodium carbonate and 50mL water were stirred until completely dissolved, 1.47g (15mmol) mesityl oxide was added, stirred at 25°C for 12 hours, extracted with dichloromethane, and spin-dried to obtain a white powdery solid, which was obtained by column chromatography 4-( 4-(5-(((2-methyl-4-oxopent-2-yl)aminomethyl)-2-oxo-3-oxazolidinyl)phenyl)-3-morpholinone.
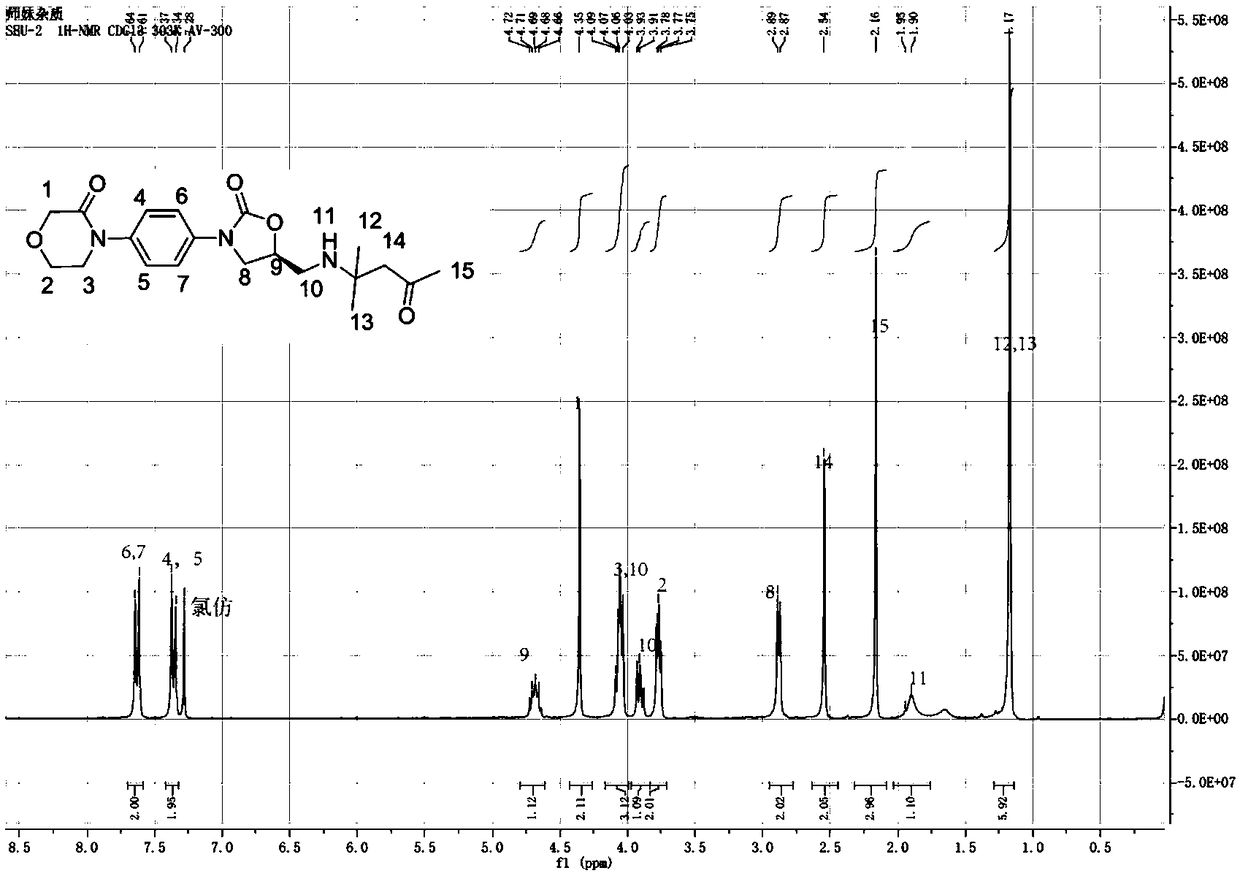
[0029] figure 1 is 4-(4-(5-(((2-methyl-4-oxopent-2-yl)aminomethyl)-2-oxo-3-oxazolidinyl)phenyl)-3- The proton nuclear magnetic resonance spectrum figure of morpholinone intermediate, by figure 1 It can be seen that this example successfully synthesized 4-(4-(5-(((2-methyl-4-oxopent-2-yl)aminomethyl)-2-oxo-3-oxazolidinyl ) phenyl) -3-morpholinone intermediate.
[0030] 2) Add 0.1g (0.25mmol) 4...
Embodiment 2
[0034] The preparation method of the rivaroxaban mesopropylidene impurity reference substance is as follows:
[0035] 1) Take 4.5g (15mmol) of 4-(4-(5-(aminomethyl)-2-oxo-3-oxazolidinyl)phenyl)-3-morpholinone, add 3.2g (30mmol) Sodium bicarbonate and 50mL of water were stirred until completely dissolved, 2.93g (30mmol) of mesityl oxide was added, stirred at 25°C for 12 hours, extracted with dichloromethane, and spin-dried to obtain a white powdery solid, which was obtained by column chromatography 4- (4-(5-(((2-methyl-4-oxopent-2-yl)aminomethyl)-2-oxo-3-oxazolidinyl)phenyl)-3-morpholinone .
[0036] figure 1 is 4-(4-(5-(((2-methyl-4-oxopent-2-yl)aminomethyl)-2-oxo-3-oxazolidinyl)phenyl)-3- The proton nuclear magnetic resonance spectrum figure of morpholinone intermediate, by figure 1 It can be seen that this example successfully synthesized 4-(4-(5-(((2-methyl-4-oxopent-2-yl)aminomethyl)-2-oxo-3-oxazolidinyl ) phenyl) -3-morpholinone intermediate.
[0037] 2) Add 0.1g (0...
Embodiment 3
[0041] The preparation of rivaroxaban isopropylidene impurity reference substance is as follows:
[0042] 1) Take 4.5g (15mmol) 4-(4-(5-(aminomethyl)-2-oxo-3-oxazolidinyl)phenyl)-3-morpholinone, add 1.2g (30mmol) Sodium hydroxide and 50mL of water were stirred until they were completely dissolved, 2.93g (30mmol) of mesityl oxide was added, stirred at 25°C for 12 hours, extracted with dichloromethane, and spin-dried to obtain a white powdery solid, which was obtained by column chromatography 4- (4-(5-(((2-methyl-4-oxopent-2-yl)aminomethyl)-2-oxo-3-oxazolidinyl)phenyl)-3-morpholinone .
[0043] figure 1 is 4-(4-(5-(((2-methyl-4-oxopent-2-yl)aminomethyl)-2-oxo-3-oxazolidinyl)phenyl)-3- The proton nuclear magnetic resonance spectrum figure of morpholinone intermediate, by figure 1It can be seen that this example successfully synthesized 4-(4-(5-(((2-methyl-4-oxopent-2-yl)aminomethyl)-2-oxo-3-oxazolidinyl ) phenyl) -3-morpholinone intermediate.
[0044] 2) Add 0.1g (0.25mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com