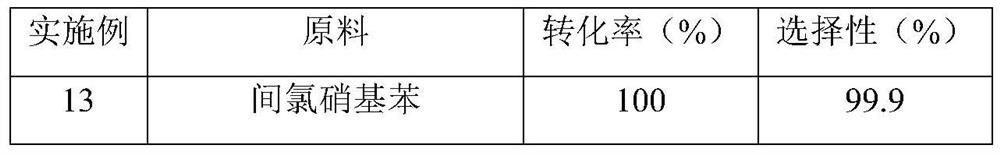
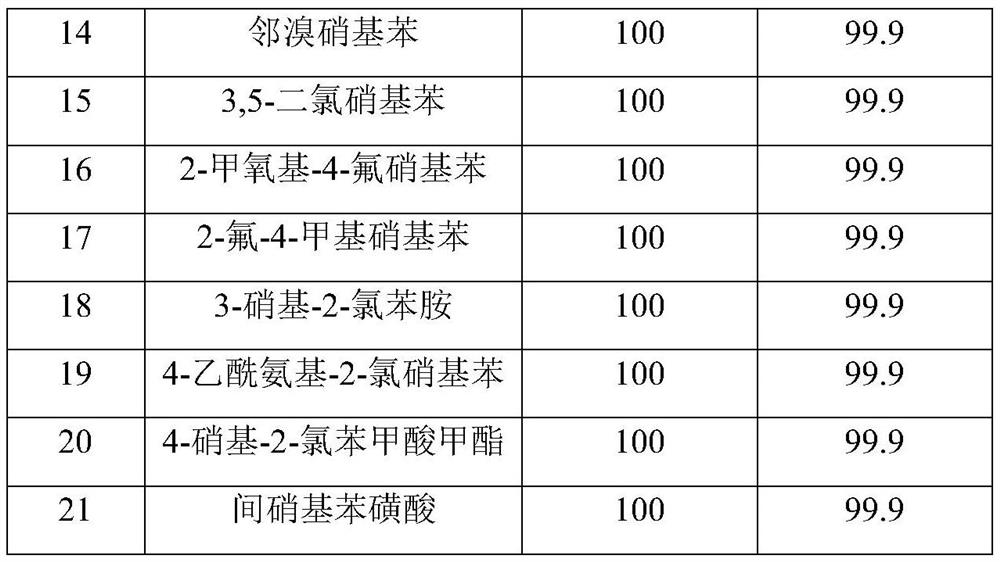
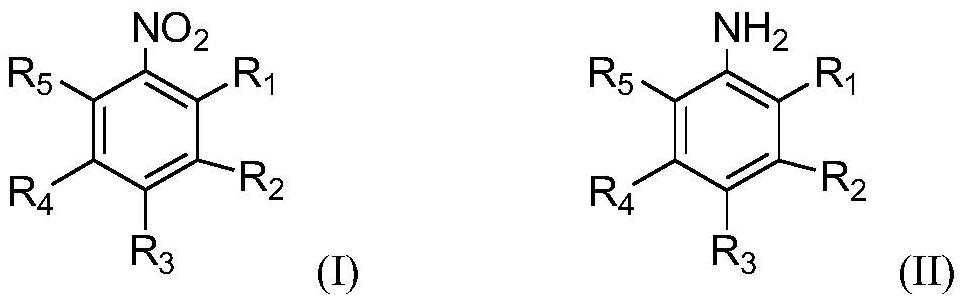Preparation method and application of a sulfur-modified activated carbon-supported noble metal catalyst
A noble metal catalyst and activated carbon technology, which is applied in the field of preparation of sulfur-modified activated carbon-supported noble metal catalysts, can solve the problems of low activity of noble metal sulfide catalysts, and achieve the effect of improving selectivity, increasing selectivity, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Weigh 10g of activated carbon with a particle size of 800 mesh and a specific surface area of 1000m 2 / g, the pore volume is 0.6ml / g, configure 6ml containing Na 2 S solution (the concentration of S in the solution is 0.0333g / ml, and the S content is 0.2g); the impregnated activated carbon is vacuum-dried at 100°C for 10h.
[0031] (2) Configure 6ml containing H 2 PdCl 4 (the concentration of Pd in the solution is 0.0333g / ml, and the Pd content is 0.2g), and the solution is added dropwise to the activated carbon loaded with sulfur obtained in step (1), and stirred evenly. The impregnated catalyst was vacuum-dried at 60 °C for 8 h.
[0032] (3) the activated carbon of the loaded sulfur and noble metal that step (2) obtains, in H 2 The reduction was carried out at 150° C. for 3 hours under the atmosphere, that is, the sulfur-modified activated carbon supported palladium catalyst.
Embodiment 2
[0034] (1) Weigh 10g of activated carbon with a particle size of 100 mesh and a specific surface area of 2000m 2 / g, the pore volume is 0.8ml / g, configure 8ml containing K 2 S solution (the concentration of S in the solution is 0.025g / ml, and the S content is 0.2g); the impregnated activated carbon is vacuum-dried at 150°C for 6h.
[0035] (2) Configure 8ml containing H 2 PtCl 6 (the concentration of Pt in the solution is 0.05g / ml, and the Pt content is 0.4g), and the solution is added dropwise to the activated carbon loaded with sulfur obtained in step (1), and stirred evenly. The impregnated catalyst was vacuum-dried at 80 °C for 4 h.
[0036] (3) the activated carbon of the loaded sulfur and noble metal that step (2) obtains, in H 2 The reduction was carried out at 120°C for 5 hours under the atmosphere, that is, the platinum catalyst supported on sulfur-modified activated carbon.
Embodiment 3
[0038] (1) Weigh 10g of activated carbon with a particle size of 1000 mesh and a specific surface area of 600m 2 / g, the pore volume is 0.3ml / g, configure 3ml containing Na 2 S solution (the concentration of S in the solution is 0.01g / ml, and the S content is 0.03g); the impregnated activated carbon is vacuum-dried at 200°C for 4h.
[0039] (2) Configure 3ml containing Pd(NO 3 ) 2solution (the concentration of Pd in the solution is 0.0333g / ml, and the Pd content is 0.1g), and the solution is added dropwise to the activated carbon loaded with sulfur obtained in step (1), and stirred evenly. The impregnated catalyst was vacuum dried at 40°C for 10 h.
[0040] (3) The activated carbon loaded with sulfur and noble metals obtained in step (2) was reduced for 10 h at 80° C. under H2 atmosphere, that is, the sulfur-modified activated carbon supported palladium catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com