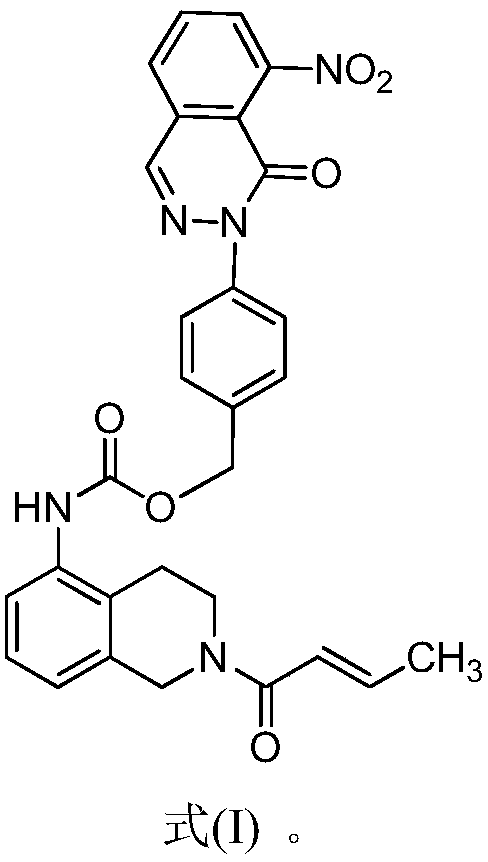
Taxol and novel nitrophthalazinone BTK inhibitor combined pharmaceutical composition and application thereof
A technology of nitrophthalazinone and inhibitors, which is applied in the field of medicinal chemistry and can solve problems such as unsatisfactory selectivity, drug resistance, and multiple side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
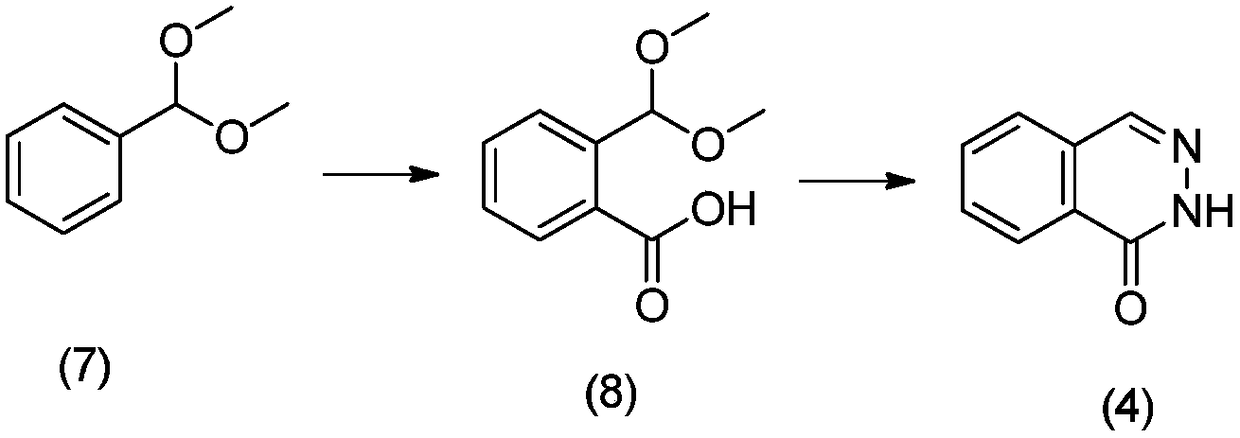
[0027] Example 1 Preparation of 8-nitro-2H-phthalazin-1-one
[0028]
[0029] Step 1: Weigh 3-nitro-1-dimethoxymethylbenzene (500mmol) into a reaction flask, add tetrahydrofuran (800ml) to dissolve, add s-BuLi (565mmol) under nitrogen protection at 60°C, and The reaction solution was stirred at -60 °C for 1 h.
[0030] Step 2: Weigh dry ice (50mmol) into a reaction flask, add tetrahydrofuran (200ml), add n-BuLi (5ml), stir for 2h under nitrogen protection, add the mixture of step 1, continue stirring for 30min, stop the reaction, add water 1000ml, adjust the pH to 2 with concentrated hydrochloric acid, separate the organic phase, extract the aqueous phase with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and recrystallize to obtain 3-nitro-2-dimethoxy methylbenzoic acid.
[0031] Step 3: Weigh the product obtained in Step 2 (400mmol), acetic acid (93mmol), and hydrazine (600mmol) into a reaction flask, add 300ml ...
Embodiment 2
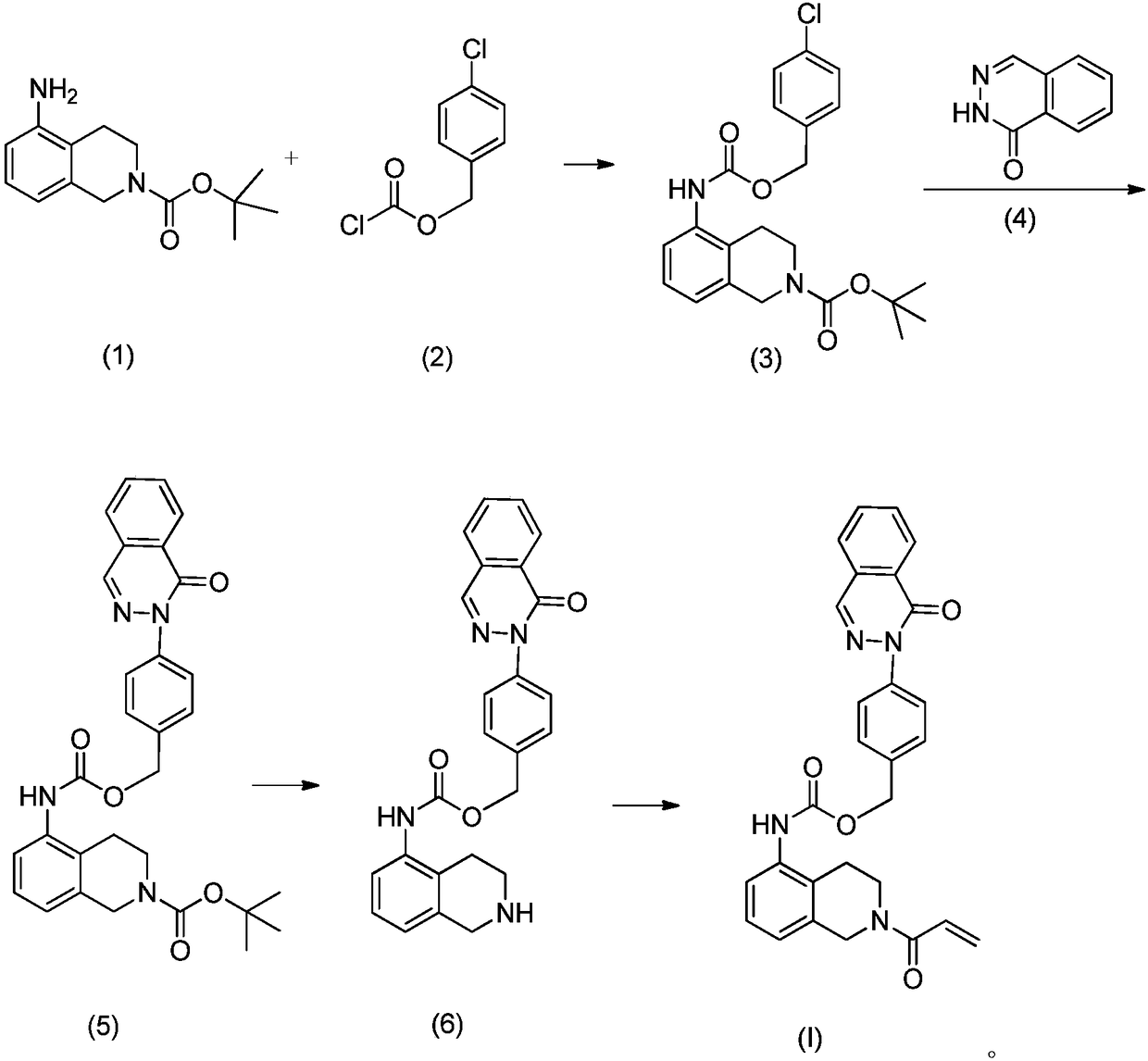
[0033] Example 2 Preparation of (3,4-dihydroisoquinoline-2(1H)-formic acid tert-butyl ester-5-yl)-carbamic acid p-chlorobenzyl ester
[0034]
[0035] Weigh triphosgene (5mmol) into a reaction bottle, add 100ml of toluene, add dropwise 20ml of tetrahydrofuran solution dissolved with p-chlorophenol (5mmol) and pyridine (10ml) at 0°C, after the drop is completed, continue to react at room temperature for 8h, concentrate the reaction solution, added 40ml of dichloromethane, suspended to dryness, and obtained p-chlorobenzyl chloroformate, which was directly used in the next step.
[0036]Weigh 5-amino-3,4-dihydroisoquinoline-2(1H)-tert-butyl carboxylate (50mmol) and DIPEA (100mmol) into a reaction flask, add 300ml of dichloromethane, and slowly add it dropwise under stirring at room temperature p-chlorobenzyl chloroformate (51mmol), after dropping, continue to stir at room temperature for 1h, stop the reaction, concentrate the reaction mixture, add 70ml of ethyl acetate, wash w...
Embodiment 3
[0038] Example 3 (3,4-dihydroisoquinoline-2(1H)-tert-butyl formate-5-yl)-carbamic acid-4-[8-nitro-(2H)-phthalazin-1-one Preparation of base] benzyl ester
[0039]
[0040] Weigh 8-nitro-2H-phthalazin-1-one and (3,4-dihydroisoquinoline-2(1H)-formic acid tert-butyl ester-5-yl)-carbamic acid p-chlorobenzyl ester (195mmol ) into the reaction bottle, add DMF100ml, react overnight at 55°C, stop the reaction, add water 100ml, dichloromethane 200ml, extract, separate the organic phase, continue to extract the water phase with dichloromethane (3*50ml), combine the organic phase , dried over anhydrous sodium sulfate, and purified by column chromatography to obtain the title compound.
[0041] ESI–MS:[M+H] + m / z 572.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com