Preparation method of Helanadizine
A technology of Hylandizine and Tetrandrine, which is applied in the field of Hylandizine preparation, can solve the problems of unreported Hylandizine recovery rate, difficulty in realizing industrialized production, and increased cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
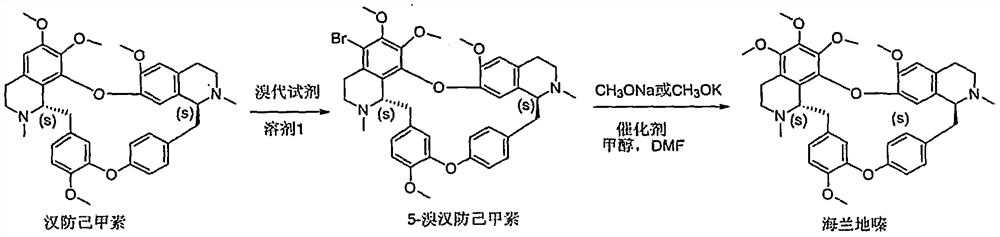
[0030] Embodiment 1: Preparation of 5-bromotetrandrine
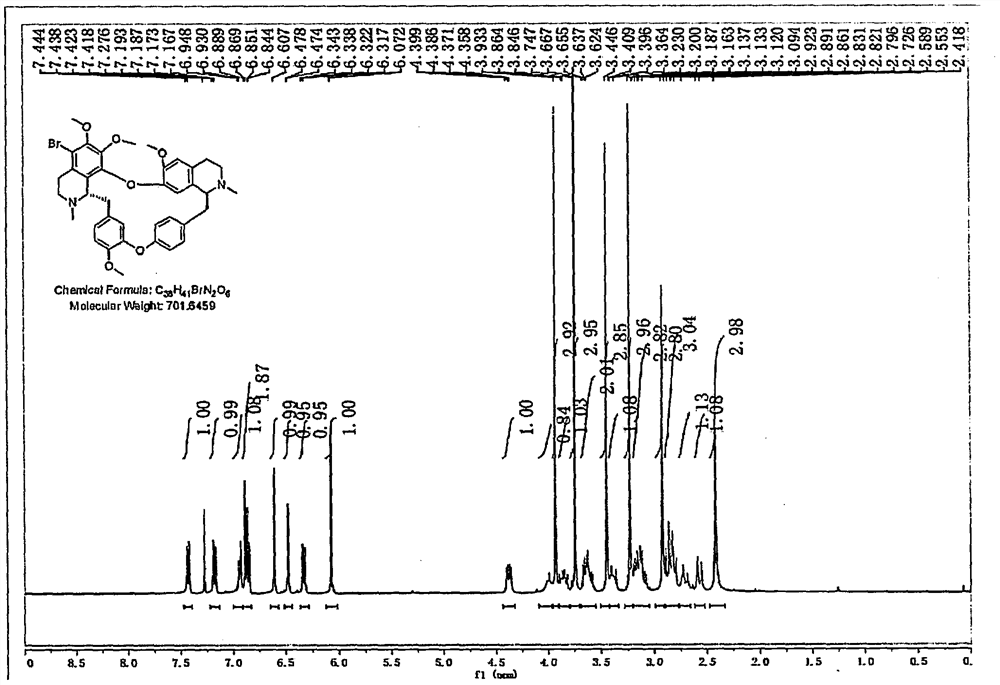
[0031] Tetrandrine is used as raw material, and tetrandrine is used as raw material, and brominated reagent is used in solvent 1 to react to obtain 5-bromotetrandrine. Among them, solvent 1 is an acidic solvent, which is liquid under normal temperature and pressure. These solvents can be used alone or mixed in any proportion; brominated reagents are N-bromosuccinimide, 1,3-dibromo- 5,5-Dimethylhydantoin, bromine simple substance.
[0032] Reactions 1 to 3 in Table 1 are the reaction yields obtained using different brominated reagents; Reaction 4 and Reaction 5 are the yields of ten grams and more of the amplified reaction using N-bromosuccinimide as the brominated reagent :
[0033] Table 1. Bromination reactions of different brominated reagents
[0034] Reaction ID Tetrandrine dosage (grams) brominated reagent Yield (%) 1 0.5 N-Bromosuccinimide 82 2 0.5 1,3-Dibromo-5,5-dimethylhydantoin...
Embodiment 2
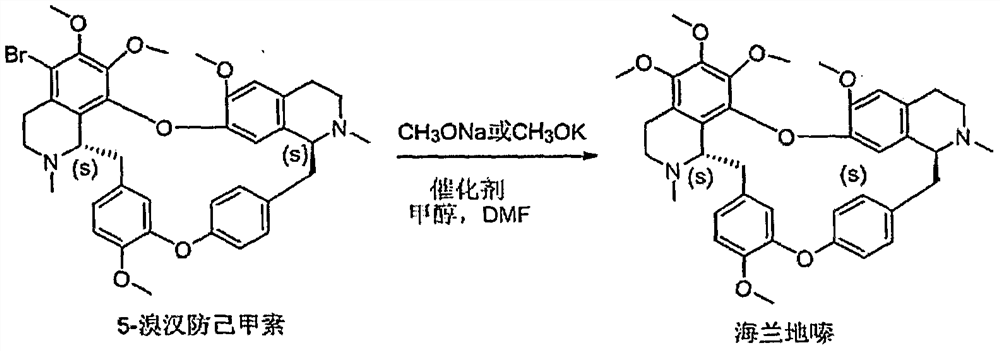
[0037] Embodiment 2: the preparation of Helanadizine
[0038] Using 5-bromotetrandrine as raw material to prepare Helanidizine, the reaction process is as follows figure 1 shown.
[0039] 5-Bromotetrandrine in N,N-dimethylformamide and methanol, under the action of a catalyst, undergoes a substitution reaction with sodium methoxide or potassium methoxide to obtain helanadizine; the catalyst is monovalent copper salt or its composite substances, such as cuprous chloride, cuprous bromide, cuprous iodide, Cu 2 Cl 2 -DMF complex, Cu 2 Cl 2 -CO 2 compound etc.
[0040] Reactions 1 to 3 in Table 2 are the reaction yields obtained by using different catalysts to prepare hylanidizine with 5-bromotetrandrine; reactions 4 and 5 are gram-scale amplification reaction yields:
[0041] Table 2. Methoxylation reactions of different catalysts
[0042] Reaction ID Dosage of 5-bromotetrandrine (g) catalyst Yield (%) 1 0.5 Cuprous Bromide 85 2 0.5 Cuprous i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com