Nanoparticles for chemotherapy, targeted therapy, photodynamic therapy, immunotherapy, and any combination thereof
A nanoparticle, immunotherapy technology, applied in biochemical equipment and methods, drug combinations, active ingredients of phosphorus compounds, etc., can solve problems such as difficult delivery of anticancer agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0335] Prodrug Synthesis
[0336] Several anticancer drugs such as etoposide (ET), paclitaxel (PTX), dihydroartemisinin (DHA) and NLG919 are conjugated to cholesterol, while camptothecin (CPT) is conjugated to oleic acid via a disulfide bond ( OA). Synthesis of prodrugs can involve conversion of hydroxyl groups in lipids (Chol) or anticancer drugs (CPT) to acid chlorides, which are then combined with drugs that also have one or more hydroxyl groups (ET, PTX, NLG919) or lipids (OA) Direct conjugation. Anticancer drugs can be prepared by functionalizing first with bis(2-hydroxyethyl) disulfide (OH-S-S-OH), followed by lipid conjugation (Chol-ET, Chol-PTX, and Chol-NLG919) or first with OH -S-S-OH modification of lipids followed by anticancer drug conjugation (OA-CPT) to introduce disulfide bonds into prodrugs.
[0337]
[0338] Scheme 3. Synthesis of Chol-S-S-OH.
[0339] Chol-S-S-OH
[0340] Cholesterol (1 eq) and 4-N,N-dimethylaminopyridine (DMAP, 4 eq) in anhydrous ...
Embodiment 2
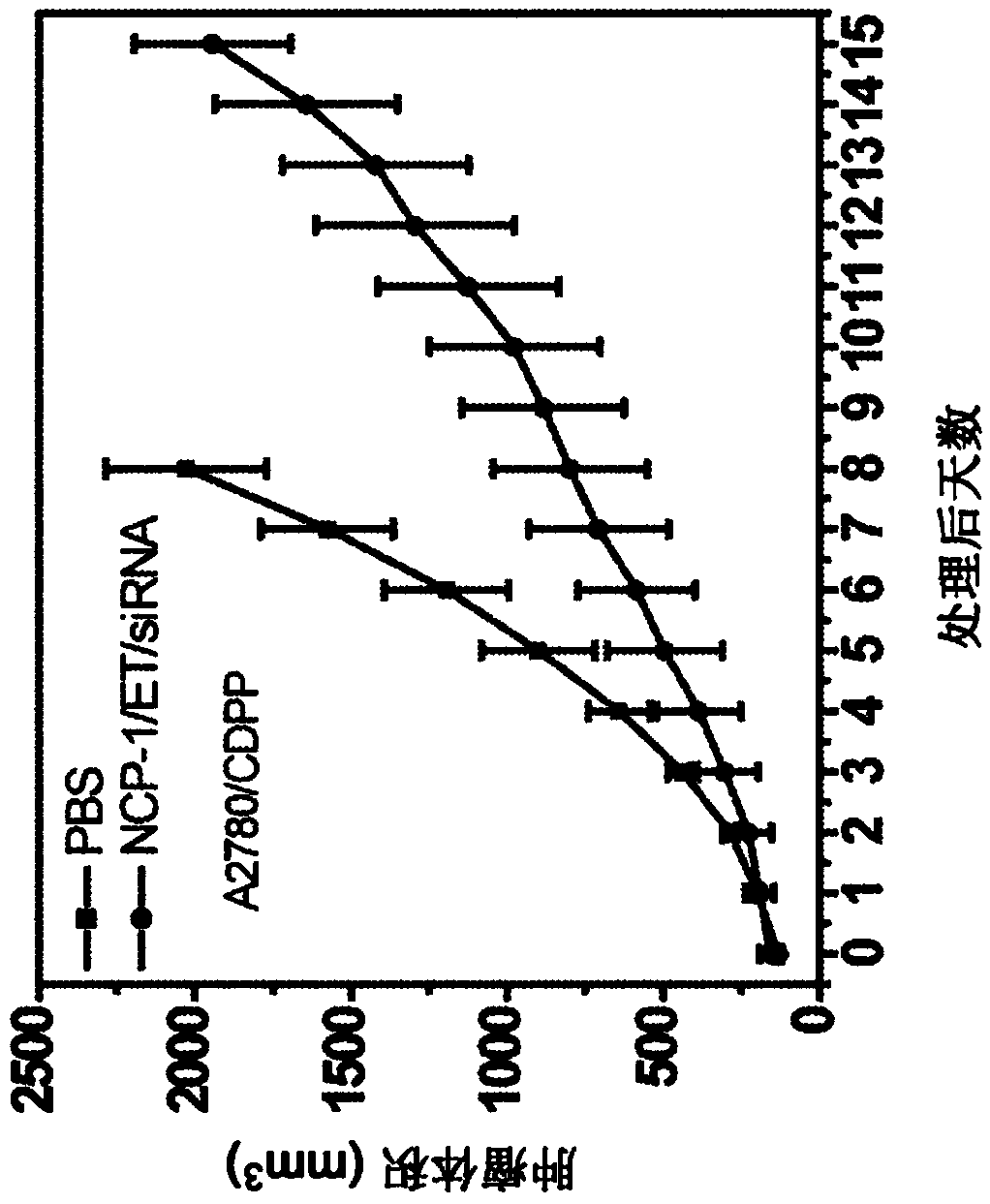
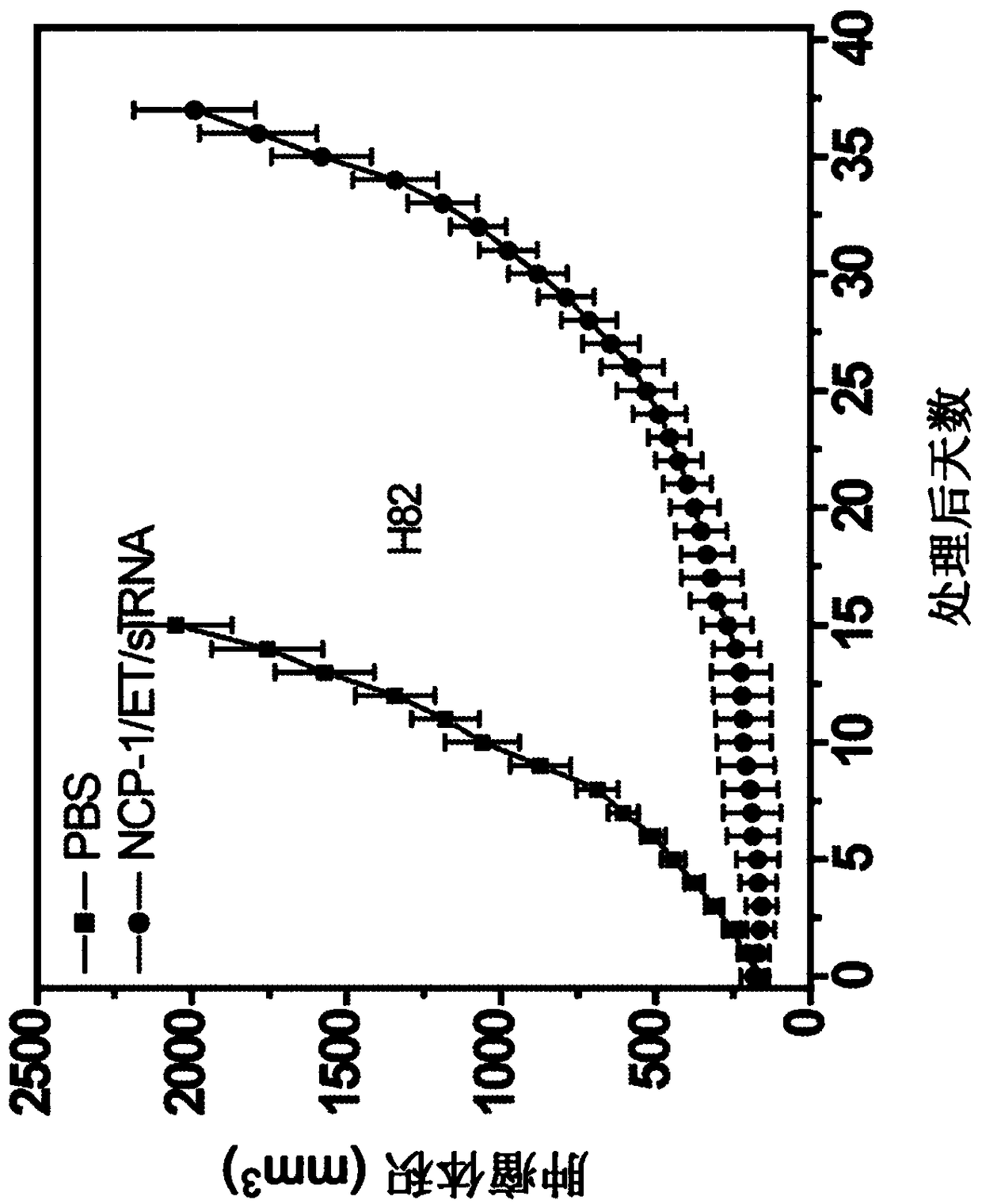
[0385] Nanoscale coordination polymer core-shell nanoparticles loaded with etoposide for the treatment of small cell lung cancer
[0386] Synthesis of Zinc Pyrophosphate Particles
[0387] First by adding 4 mL of Na 4 P 2 o 7 10H 2 O (25 mg / mL in water) and 4 mL of Zn (NO 3 ) 2 ·6H 2 O (100 mg / mL in water) forms a microemulsion. The individual microemulsions were stirred vigorously at room temperature for 15 min, and 400 μL of DOPA solution (200 mg / mL of CHCl 3 solution) added to Na 4 P 2 o 7 10H 2 O solution and continued to stir for 15 minutes until a clear solution formed. Then, Zn(NO 3 ) 2 ·6H 2 O solution was slowly added to Na 4 P 2 o 7 10H 2 O solution, the combined solution was allowed to react at room temperature for 30 minutes. The pellet was pelleted after adding 400 mL of ethanol and obtained by centrifugation at 12000 rpm for 30 minutes. The resulting precipitate was further washed once with 50% EtOH / cyclohexane, twice with 50% EtOH / THF, and...
Embodiment 3
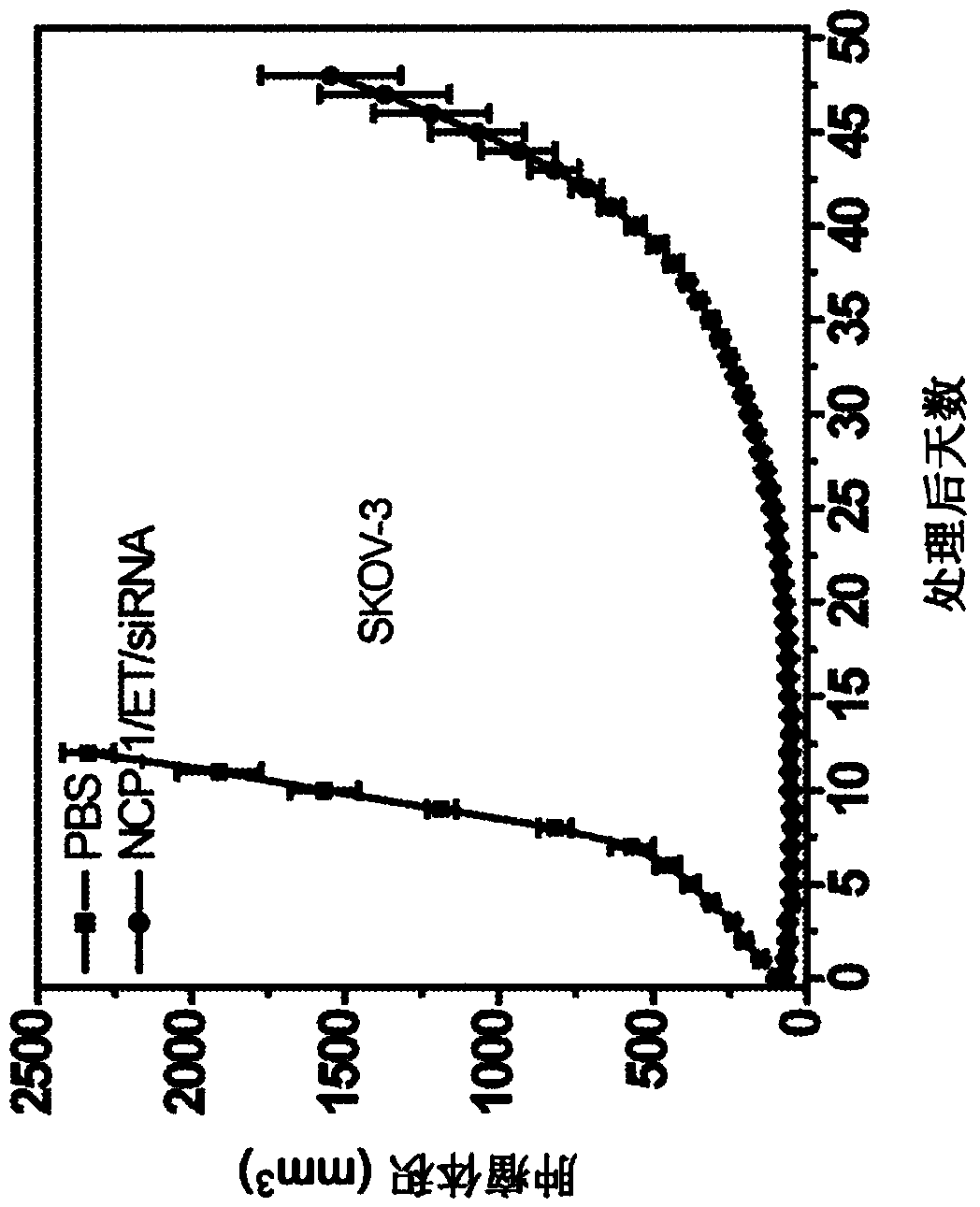
[0398] Paclitaxel-loaded nanoscale coordination polymer core-shell nanoparticles for the treatment of non-small cell lung cancer
[0399] Preparation and characterization of Zn / PTX.
[0400] At 60°C, add 80 μL of THF solution of DSPC, cholesterol, Chol-PTX (molar ratio 1:1:0.3), DSPE-PEG2k (20 mol%) and zinc pyrophosphate bare particles to 500 μL of 30% (v / v)EtOH / H 2 O to obtain Zn / PTX. THF and EtOH were evaporated and the solution was cooled to room temperature before use. The particle size and distribution were determined by DLS, the Z-average diameter was 94.15±0.61 nm, the number-average diameter was 55.83±6.20 nm, the PDI was 0.119±0.01, and the ζ-potential was -0.67±1.02 mV. The drug loading of Zn / PTX particles was 7.14%.
[0401] In vitro cytotoxicity against non-small cell lung cancer cells
[0402] H460 and A549 cells seeded on 96-well plates at a density of 2000 cells / well were treated with Zn / PTX and free PTX at various etoposide concentrations for 72 hours....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com