A sort of 18 F-labeled benzimidazole compound and its preparation method and application
A compound and synthesis method technology, applied in the field of medicine, can solve the problems of rare radiolabeling and imaging, and achieve the effects of good industrial application prospects, simple and easily controllable preparation process, and high specific activity of radioactivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
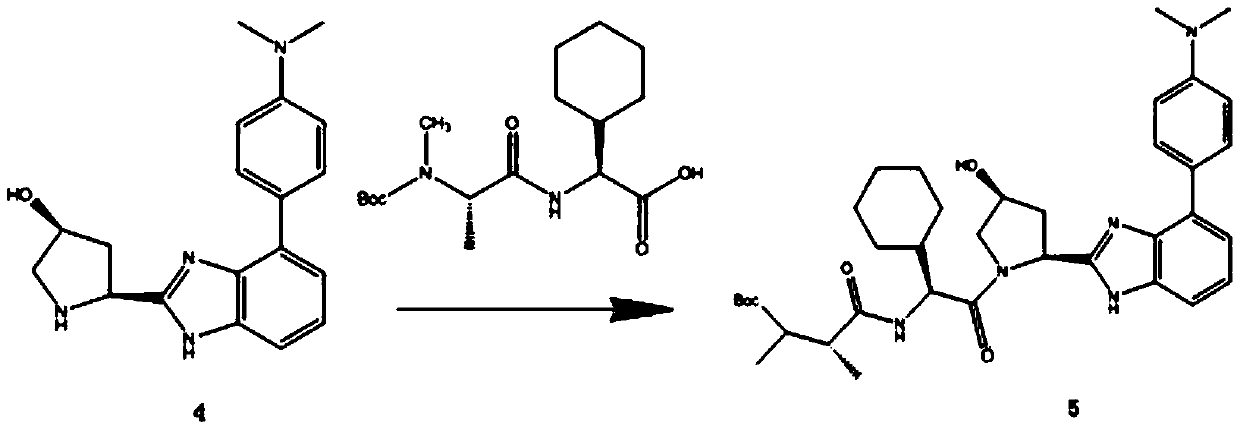
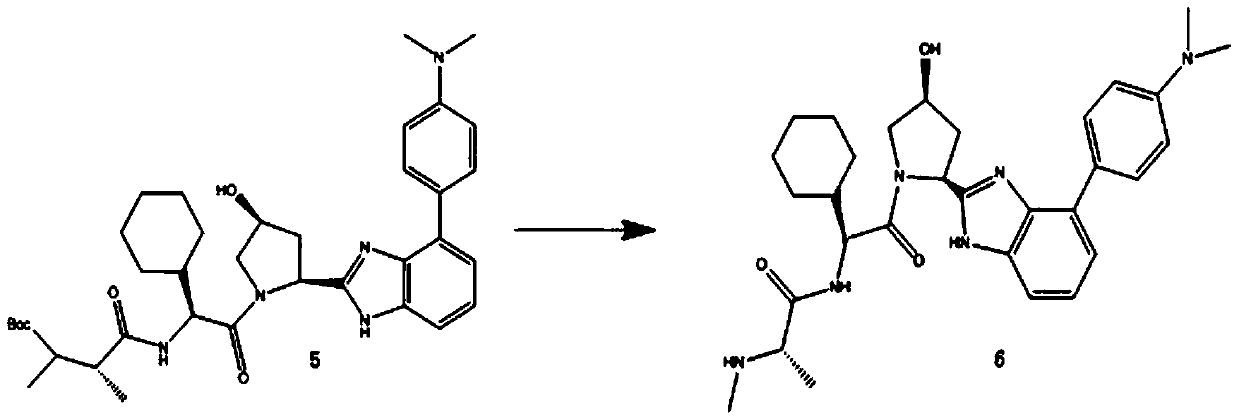
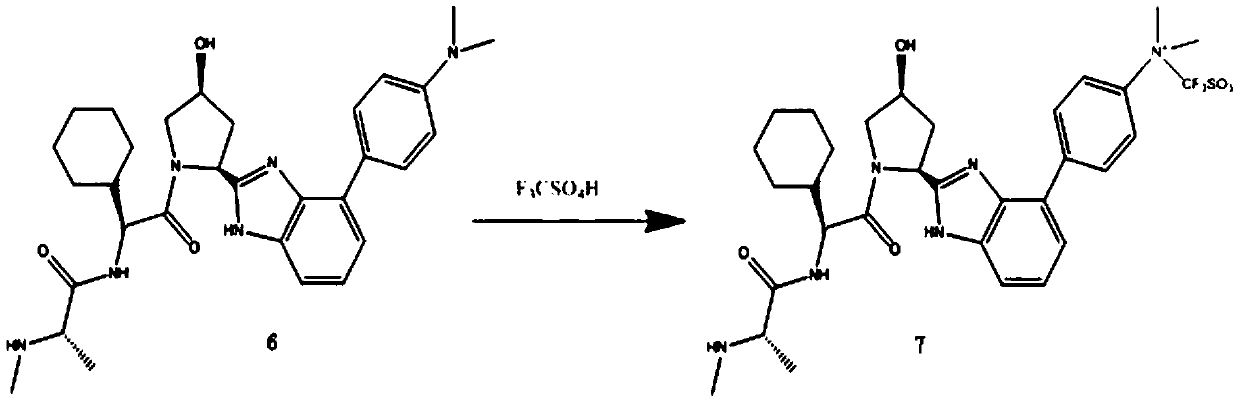
[0056] In view of this, in a typical embodiment of the present invention, a kind of 18 The synthetic method of 2-[(1-methyl-1-methylamino) carbonamide-1-cyclohexyl-carbonyl-3-hydroxyl-pyrrole]-3-(4-fluoro-phenyl)-imidazole of F mark, The method comprises the following reaction steps:
[0057] (1)
[0058] (2)
[0059] (3)
[0060] (4)
[0061]
[0062] (5)
[0063]
[0064] (6)
[0065]
[0066] (7)
[0067]
[0068] In yet another specific embodiment of the present invention, the specific method of the step (1) is:
[0069] Dissolve 2-methyl-3-N,N-dimethylnitrobenzene in the mixed solution of methanol-water, add ammonium chloride, add Zn under ice bath, filter, concentrate the filtrate under reduced pressure, add ethyl acetate , dried to obtain intermediate 2;
[0070] In yet another specific embodiment of the present invention, the molar ratio of 2-methyl-3-N,N-dimethylnitrobenzene to ammonium chloride is 1:0.5-2; the volume ratio of methanol to wa...
Embodiment 1
[0093] Dissolve 2.6g of 2-methyl-3-N,N-dimethylnitrobenzene in a mixed solution of 50ml of methanol-water (volume ratio 1:1), add 0.2g of ammonium chloride, and add 1.5 g Zn, filtered, the filtrate was concentrated under reduced pressure, added ethyl acetate, washed with water, and dried over anhydrous sodium sulfate to obtain intermediate 2; ESI-MS (m / z): 227 (M + ); 1 H NMR (DMSO-d 6, , 400MHz) δ:8.79(s,4H,2×NH 2 ),6.39-7.41(m,7H,Ar-H) 13 , 2.65(s,6H,NCH 3 ×2) CNMR (CDCl 3 ,125MHz)δ,145.7,134.3,133.5,129.6,129.6,125.5,1 24.2,118.3,117.6,111.2,111.2,112.5,42.7,42.8; the reaction formula is as follows:
[0094]
Embodiment 2
[0096] Dissolve 3.5g of N-Cbz-protected proline derivatives and 2.5g of N-methylmorpholine in 30ml of dry dichloromethane, add 5ml of isobutyl chloroformate dropwise, stir, and dissolve 3.8g of intermediate 2 in 10ml In dichloromethane, add dropwise to the above system, react at low temperature, and rise to room temperature to obtain intermediate 3; ESI-MS (m / z): 474 (M + ); 1 H NMR (DMSO-d 6, , 400MHz) δ: 13.75(s, H, OH), 8.25(s, 2H, NH 2 ), 7.85(s,H,NH),6.12-7.68(m,12H,Ar-H) 13 ,2.11-3.41(d,4H,CH 2 ),5.56(S,2H,CH 2 ), 3.11-4. 25(t, 2H, CH), 2.65(s, 6H, NCH 3 ×2) 13 CNMR (CDCl 3 ,125MHz)δ, 169.5, 158.7, 141.4, 139.2, 128.5, 126., 7, 126.3, 125.9, 125.6, 124.5, 123.1, 122.9, 122.9, 122.6, 121.4, 120.9, 120.3, 114.6, 113.5, 123.9, 7 65.6, 55.3, 54.6, 41.6, 41.1; the reaction formula is as follows:
[0097]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com