Synthetic method of 3-bromo-5-aryl-2-(trimethylsilyl)-1-(N,N-dimethylsulfonamide)pyrrole
A technology of trimethylsilyl and dimethylsulfonyl, applied in the field of regioselective synthesis of 3-bromo-5-aryl-2--1-pyrrole, which can solve the problem of inflexible substituents and the range of reaction substrates Narrow, complex catalyst preparation process and other problems, to achieve the effect of simple synthesis method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The first step, the synthesis of compound 1
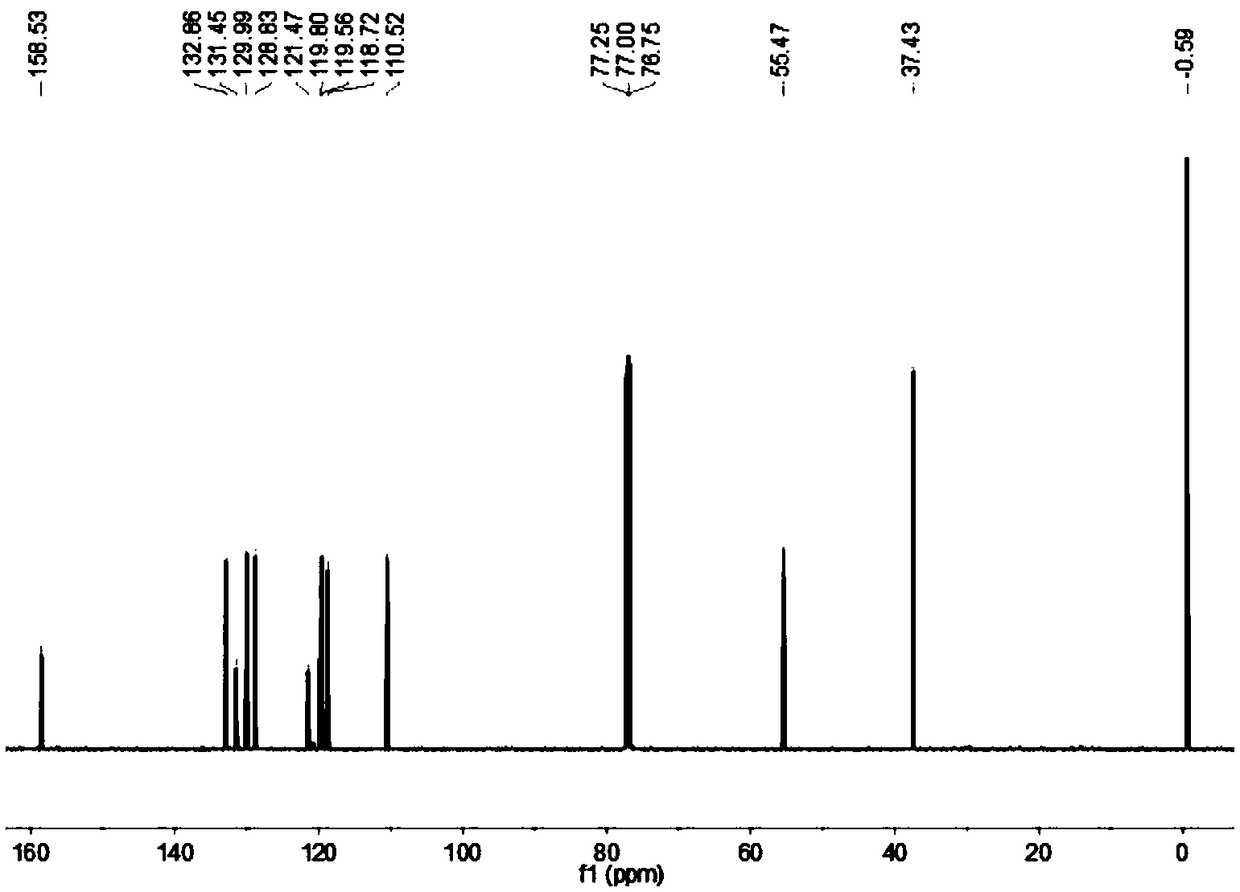
[0044] 2,5-bis(trimethylsilyl)-1-(N,N-dimethylsulfonylamino)pyrrole (2.06 g, 6.5 mmol), chloroform (20 mL) and hydrochloric acid ( 30 μL), react at room temperature for 0.5 h. Add an appropriate amount of saturated sodium bicarbonate solution to the round bottom flask, extract with ether (10mL×3), collect the organic phase, wash with water and saturated brine successively, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain a colorless liquid, which was separated and purified by column chromatography using n-hexane / diethyl ether (60:1, v / v) as the eluent to obtain 1.61 g of a white solid with a yield of 78%; mp:54-58℃; 1 H NMR (500MHz, CDCl 3 )δ7.19(d, J=1.5Hz, 1H, pyrrole-H), 6.50(d, J=1.5Hz, 1H, pyrrole-H), 2.79(s, 6H, Me-H), 0.32(s, 9H,TMS-H),0.21(s,9H,TMS-H); 13 C NMR (125MHz, CDCl 3 )δ137.3, 130.41, 128.15, 122.57, 38.47, 0.46, 0.4; HRMS (ESI-TOF) for C 6 h 10 N 2 o 2...
Embodiment 2
[0058] The first step, the synthesis of compound 1
[0059] With embodiment 1.
[0060] The second step, the synthesis of compound 2
[0061] Add compound 1 (574 mg, 1.8 mmol), NBS (480.6 mg, 2.7 mmol) and THF (25 mL) into a 50 mL two-neck flask under nitrogen protection, and stir for 1 h in an ice-water bath. Add an appropriate amount of saturated sodium thiosulfate solution to the mixture, extract with ether (15mL×3), combine the organic phases, wash with water and saturated brine successively, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain a colorless liquid, which was separated and purified by column chromatography using n-hexane / diethyl ether (60:1, v / v) as the eluent to obtain 465 mg of a colorless liquid with a yield of 80%.
[0062] The third step, synthesis of compounds 3a-3d
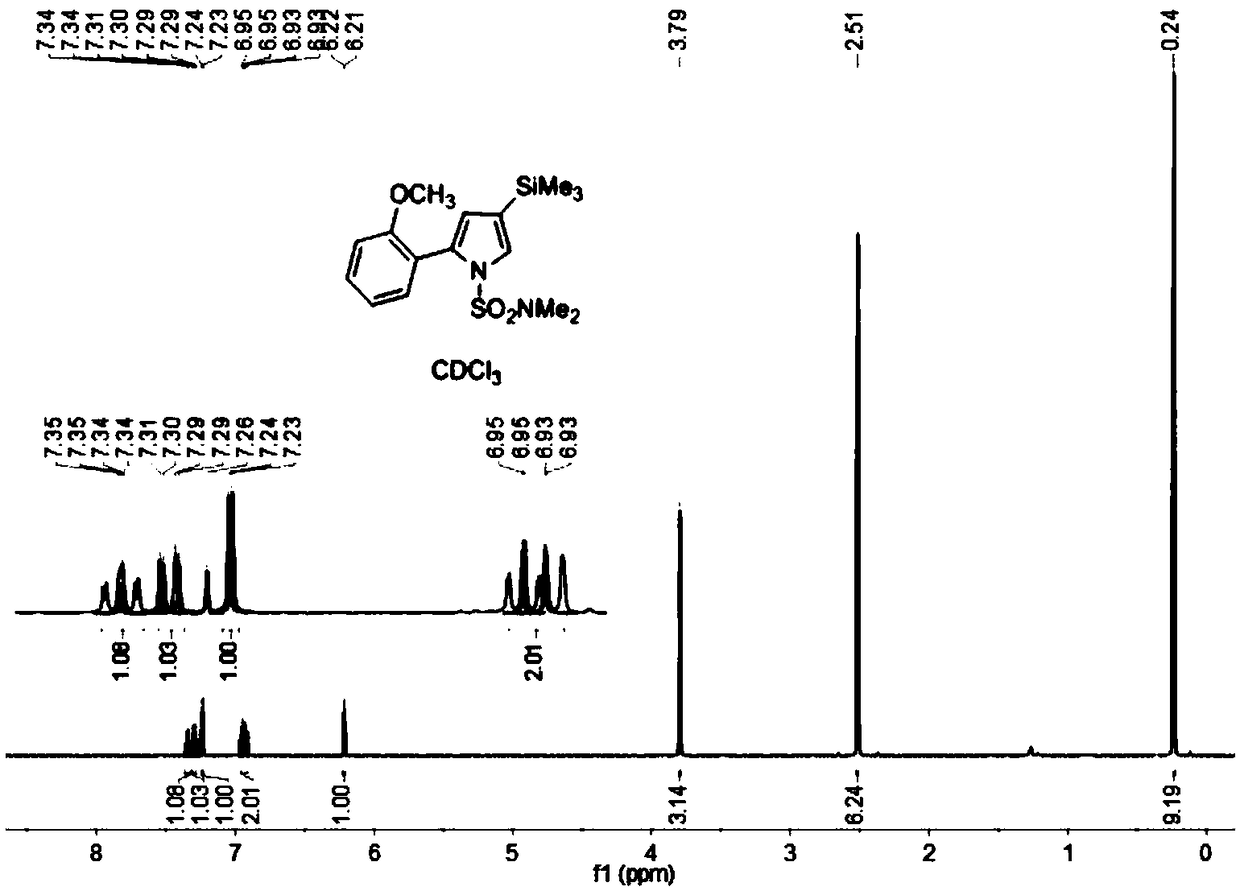
[0063] Add compound 2 (0.60mmol), o-methoxyphenylboronic acid (1.0mmol), 2M K 2 CO 3 (2mL), Pd(PPh 3 ) 4 (10mg, 0.01mmol) and DMF (6mL) we...
Embodiment 3
[0073] The first step, the synthesis of compound 1
[0074] With embodiment 1.
[0075] The second step, the synthesis of compound 2
[0076] Add compound 1 (636.3 mg, 2.0 mmol), NBS (445.0 mg, 2.5 mmol) and THF (30 mL) into a 50 mL two-neck flask under nitrogen protection, and stir at 0° C. for 1 h. Add an appropriate amount of saturated sodium thiosulfate solution to the mixture, extract with ether (20mL×3), combine the organic phases, wash with water and saturated brine successively, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain a colorless liquid, which was separated and purified by column chromatography using n-hexane / diethyl ether (60:1, v / v) as eluent to obtain 557 mg of a colorless liquid with a yield of 86%.
[0077] The third step, synthesis of compounds 3a-3d
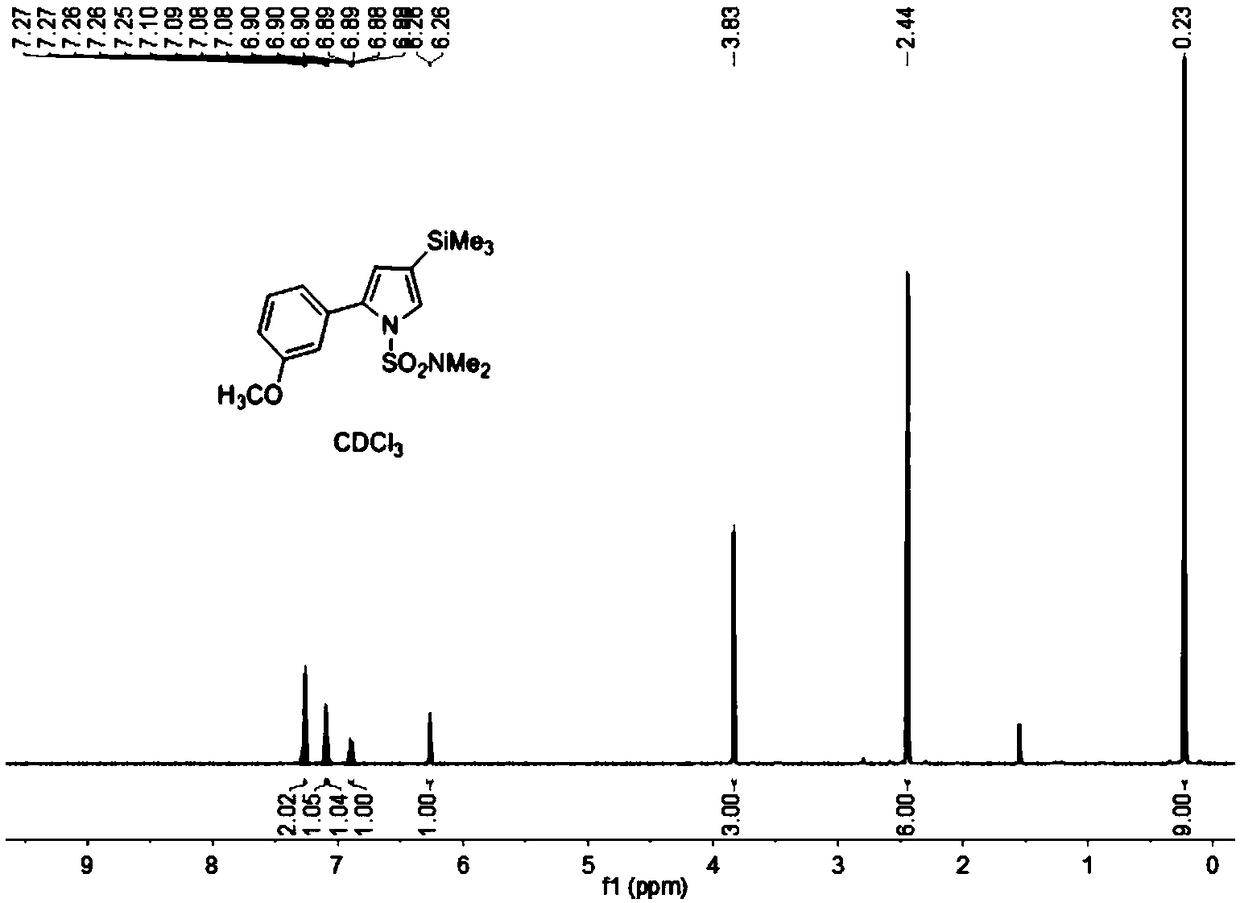
[0078] Add compound 2 (0.40mmol), o-methoxyphenylboronic acid (0.48mmol), 2M K 2 CO 3 (2mL), Pd(PPh 3 ) 4 (8 mg, 0.008 mmol) and DMF (5 mL) were used as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com