Method for extracting lithium, rubidium and co-producing zeolite or potassium nepheline from lithium mica ore
A technology of lepidolite and potassium nepheline, which is applied in the field of rubidium and by-product zeolite or potassium nepheline and extraction of lithium, can solve the problems of difficult utilization of leaching slag, high equipment requirements, equipment corrosion, etc., and achieves comprehensive utilization and equipment. Simple requirements and the effect of meeting environmental protection requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
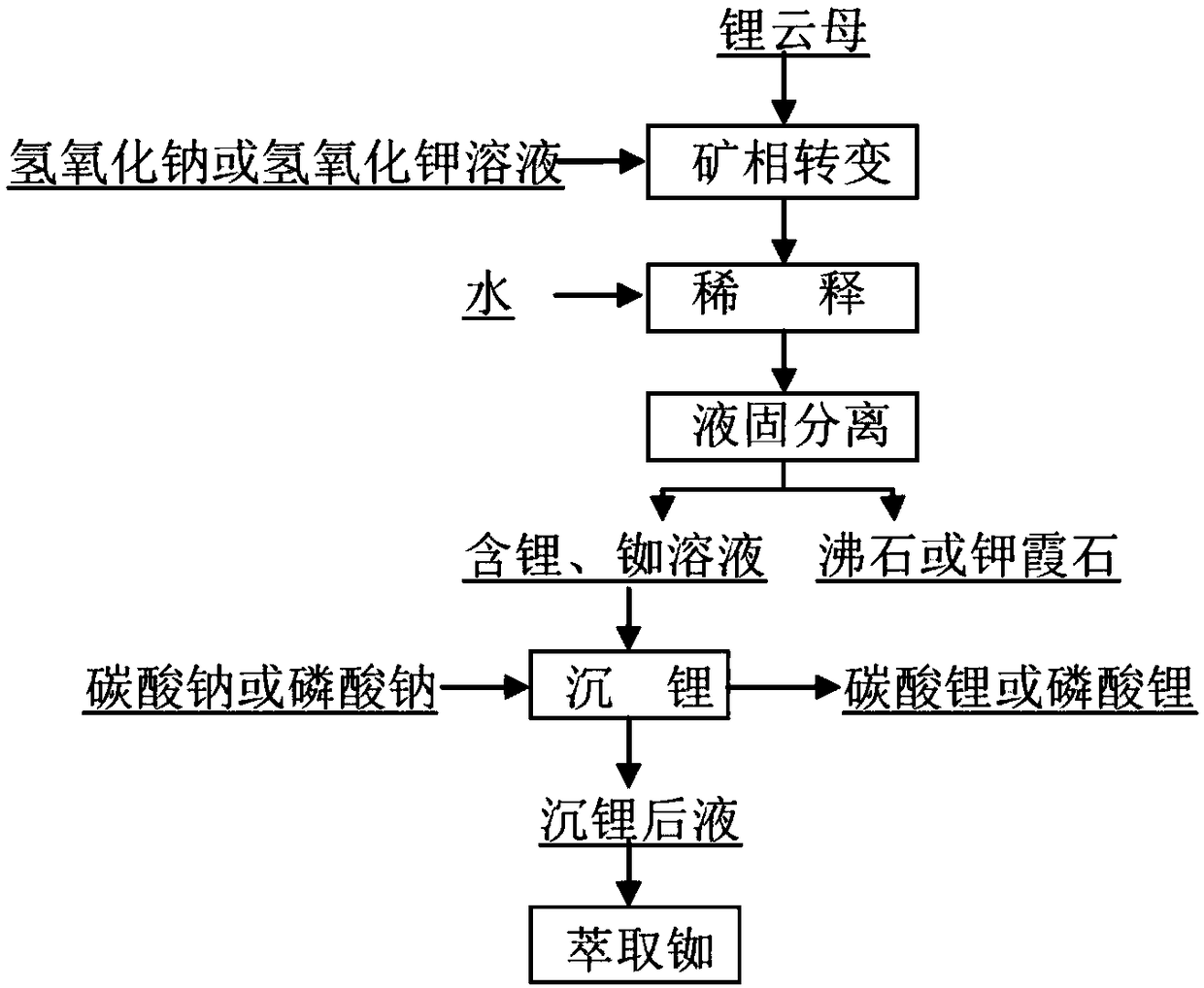
[0019] (1) 80g lepidolite ore (containing Li 2.2%, Rb 0.6%, particle size-100 mesh) is mixed with 60% sodium hydroxide solution, wherein, the weight ratio of sodium hydroxide and lepidolite ore is 4.8:1, in The temperature was 270°C for 2 hours.
[0020] (2) Dilute the pulp that has been reacted in step (1) with water, the weight of the water used for dilution is 3 times the weight of lepidolite, filter to obtain lithium-containing solution and zeolite products, the extraction rate of lithium reaches 94.2%, and the extraction rate of rubidium reaches 96.4% .
[0021] (3) The lithium-containing and rubidium-containing solution obtained in step (2) is added to sodium carbonate and undergoes a precipitation reaction to obtain lithium-precipitated liquid and lithium carbonate products.
[0022] (4) Add the lithium precipitation solution obtained in step (3) to t-BAMBP for extraction and recovery of rubidium.
Embodiment 2
[0024] (1) 80g lepidolite ore (containing Li 0.6%, Rb 0.09%, particle size-100 mesh) is mixed with 70% sodium hydroxide solution, wherein, the weight ratio of sodium hydroxide and lepidolite ore is 8:1, in The temperature was 330°C for 0.5h.
[0025] (2) Dilute the pulp that has been reacted in step (1) with water, the weight of the water used for dilution is 5 times the weight of lepidolite, filter to obtain a solution containing lithium and rubidium and a zeolite product, wherein the extraction rate of lithium reaches 99.1%, and the extraction rate of rubidium The rate reached 99.3%.
[0026] (3) adding the lithium- and rubidium-containing solution obtained in step (2) into sodium phosphate to undergo a precipitation reaction to obtain lithium-precipitated liquid and lithium phosphate products.
[0027] (4) Add the lithium precipitation solution obtained in step (3) to t-BAMBP for extraction and recovery of rubidium.
Embodiment 3
[0029] (1) 80g lepidolite ore (containing Li 1.8%, Rb 0.3%, particle size-200 mesh) is mixed with 85% sodium hydroxide solution, wherein, the weight ratio of sodium hydroxide to lepidolite ore is 2.8:1, in The temperature was 270°C for 3h.
[0030] (2) Dilute the pulp that has been reacted in step (1) with water, the weight of the water used for dilution is 5 times of the lepidolite weight, filter to obtain a solution containing lithium and rubidium and a zeolite product, wherein the extraction rate of lithium reaches 92.8%, and the extraction rate of rubidium The rate reached 94.6%.
[0031] (3) adding the lithium- and rubidium-containing solution obtained in step (2) into sodium phosphate to undergo a precipitation reaction to obtain lithium-precipitated liquid and lithium phosphate products.
[0032] (4) Add the lithium precipitation solution obtained in step (3) to t-BAMBP for extraction and recovery of rubidium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com