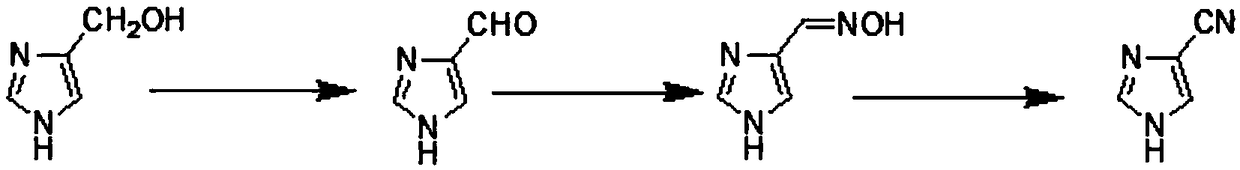
Method for preparing 1H-imidazole-4-carbonitrile
A technology of imidazole and carbonitrile, which is applied in the field of preparation of 1H-imidazole-4-carbonitrile, can solve the problems of high price, unsuitability for industrial production, and difficult acquisition of raw materials, and achieve the advantages of convenient operation, low cost and high product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] ①The preparation of 1H-imidazole-4-carbaldehyde, the steps are as follows:
[0034] In a 500ml four-necked flask, add 25.1g (0.254mol) 4-hydroxymethylimidazole and 125.5g methanol in sequence, the mass ratio of 4-hydroxymethylimidazole and methanol is 1:5, stir evenly, and add 66.3g (0.762mol) manganese dioxide, the molar ratio of 4-hydroxymethylimidazole and manganese dioxide is 1:3, after the feeding is completed, the temperature is raised to 70°C for 6 hours, then the reaction solution is cooled to 25°C, and the manganese mud is removed by filtration , the filtrate is 1H-imidazole-4-carbaldehyde reaction solution (0.229mol), the yield is 90%, and the next step is directly synthesized.
[0035] 2. The preparation of 4-methoxime imidazole, the steps are as follows:
[0036] Stir and cool down the 1H-imidazole-4-formaldehyde reaction liquid (0.229mol) obtained in step ① to 25°C, slowly add 19.1g (0.275mol) of hydroxylamine hydrochloride, the molar ratio of 1H-imidazole...
Embodiment 2
[0040] All the other steps are as follows with the preparation of embodiment 1,4-methoxyimidazole:
[0041] 2. The preparation of 4-methoxime imidazole, the steps are as follows:
[0042] Distill methanol under reduced pressure from step ① (0.229mol) 1H-imidazole-4-formaldehyde reaction solution, add 110g of pyridine, stir and cool down to 25°C, the mass ratio of 1H-imidazole-4-formaldehyde to pyridine is 1:5, slowly add hydrochloric acid Hydroxylamine 19.1g (0.275mol), the molar ratio of 1H-imidazole-4-carbaldehyde and hydroxylamine hydrochloride is 1:1.2, the temperature is controlled at 55°C, after the addition is completed, it is kept at room temperature for 2 hours, and the pyridine is distilled from the solvent to obtain a viscous kettle liquid 4-methoximylimidazole (0.227mol), yield 99%, directly synthesized to the next step.
Embodiment 3
[0044] ①The preparation of 1H-imidazole-4-carbaldehyde, the steps are as follows:
[0045]In a 500ml four-necked flask, add 25.1g (0.254mol) 4-hydroxymethylimidazole and 125.5g methanol in sequence, the mass ratio of 4-hydroxymethylimidazole and methanol is 1:5, stir evenly, and add 123.7g (1.422mol) manganese dioxide, the molar ratio of 4-hydroxymethylimidazole and manganese dioxide is 1:5.6, after the addition is completed, the temperature is raised to 40°C for 6 hours, then the reaction solution is cooled to 25°C, and the manganese mud is removed by filtration , the filtrate is 1H-imidazole-4-carbaldehyde reaction liquid (0.229mol), the yield is 92%, and the next step is directly synthesized.
[0046] All the other steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com