Application of conivaptan hydrochloride in preparation of medicine for treating feline infectious peritonitis
A conivaptan hydrochloride, infectious technology, applied in the field of preparation of drugs for the treatment of feline infectious peritonitis, can solve the problems of large side effects, can not reduce the high mortality rate of feline infectious peritonitis, and achieve high safety and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
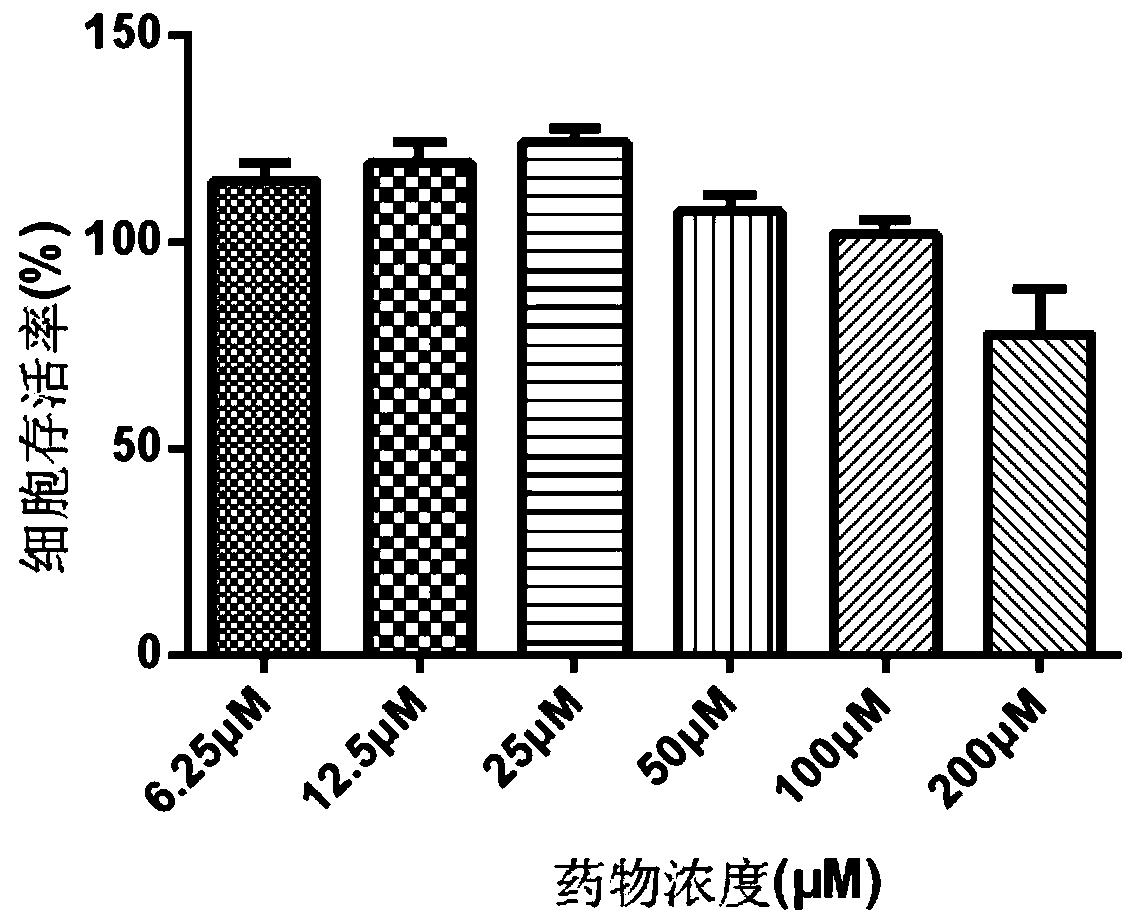
[0013] Embodiment 1 conivaptan hydrochloride is to the toxicity test of cell
[0014] 1. Test method
[0015] 1) Take well-growing CCL-94 (cat kidney cells) for digestion and passage, and use cell growth medium to adjust the cell density to 1×10 5 / mL, seeded in a 96-well plate, 100 μL per well, placed at 37°C, 5% CO 2 Cultivate in an incubator for 16 hours;
[0016] 2) After 16 hours, take out the 96-well plate, discard the culture medium in the well, wash three times with sterile PBS, and after drying, add 100 μL of cell maintenance solution to the first column of each group, and add 50 μL cell maintenance solution;
[0017] 3) Make a mark on the lid, add conivaptan hydrochloride to the first column of each group in the order of marking, perform 2-fold dilution, mix gently with a row gun for ten times, suck out 50 μL and add it to the second column, and then By analogy, mix and discard 50 μL at the end. Make the final concentration of conivaptan hydrochloride 200 μM, 10...
Embodiment 2
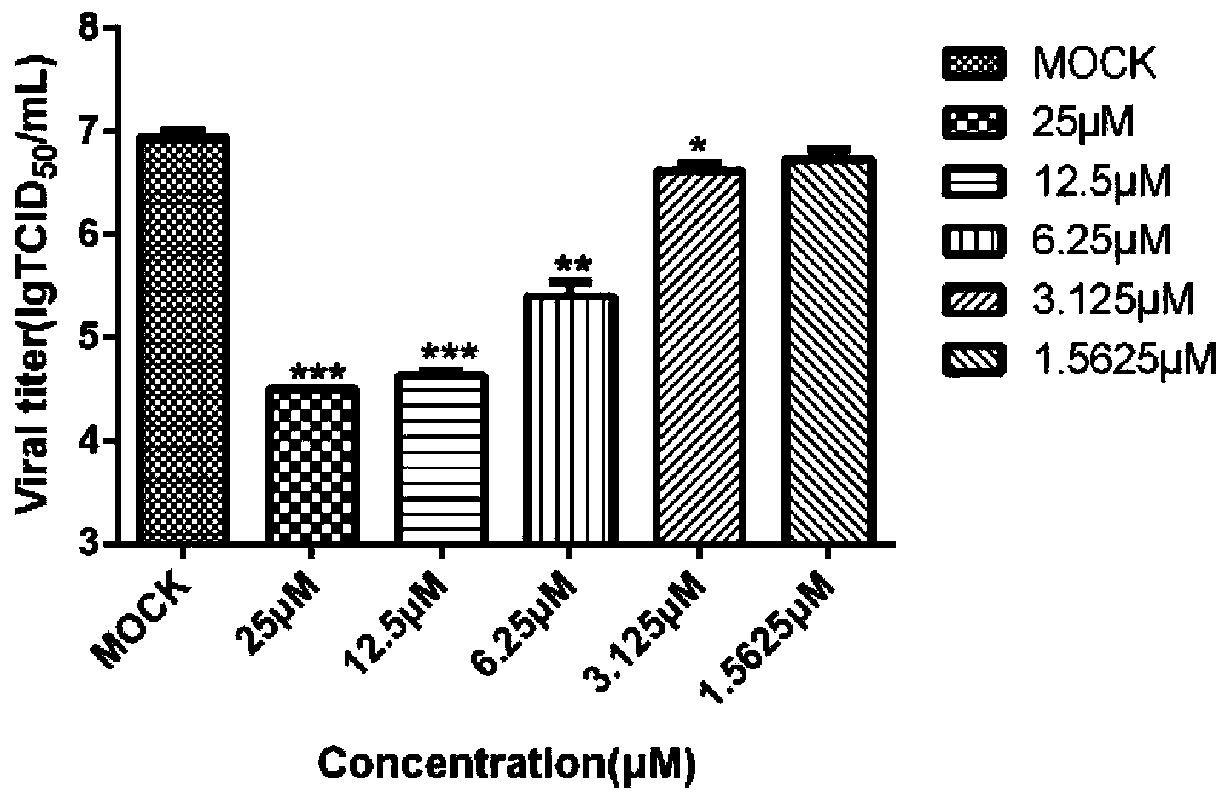
[0021] Embodiment 2 conivaptan hydrochloride is active TCID to feline coronavirus 50 Determination of
[0022] 1. Test method
[0023] 1) The CCL-94 cells were digested and passaged, and the cell density was adjusted to 1×10 with cell growth medium. 5 / mL, inoculate 2mL / well in 12-well plate, 37℃, 5%CO 2 Cultivate in an incubator for 16 hours;
[0024] 2) Take out the 12-well plate, mark it well, mix the cell maintenance solution with conivaptan hydrochloride so that the final concentrations of conivaptan hydrochloride are 25 μM, 12.5 μM, 6.25 μM, 3.125 μM, 1.5625 μM, and place on a shaker Mix for at least 5 seconds;
[0025] 3) Discard the medium in the wells of the 12-well plate, wash with sterile PBS three times, add the diluted drug after drying, and place at 37°C, 5% CO 2 Incubate for 1 hour in the incubator;
[0026] 4) After 1 hour, take out the 12-well plate, inoculate each well with 0.01 MOI of feline coronavirus, shake the 12-well plate to mix well, set virus c...
Embodiment 3
[0032] The indirect immunofluorescence detection of embodiment 3 conivaptan hydrochloride to feline coronavirus activity
[0033] 1. Test method
[0034] 1) Take CCL-94 cells in good growth state for digestion and passage, and adjust the cell density to 1×10 with cell growth medium. 5 / mL, inoculate 100 μL / well in a 96-well plate, and place at 37°C, 5% CO 2 Cultivate in an incubator for 16 hours;
[0035] 2) After 16 hours, the cells were covered with a single layer, and the 96-well plate was taken out and washed twice with serum-free DMEM medium, replaced with a cell maintenance solution, and the compound was added for 2-fold dilution. The final concentration of conivaptan was 25 μM, 12.5 μM, 6.25 μM, 3.125 μM, and 1.5625 μM, and a virus control group and a cell control group were set at the same time;
[0036] 3) When 60% of the cells have lesions, discard the medium in the well, wash with PBS twice, and then add 4% paraformaldehyde to fix the cells for 30 minutes;
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com