A kind of synthetic method of carbocyclic derivative substituted by unsaturated double bond
A synthesis method and unsaturated technology, which are applied in the synthesis field of carbocyclic derivatives, can solve the problems of low reaction stability, harsh synthesis reaction conditions, complicated reaction steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
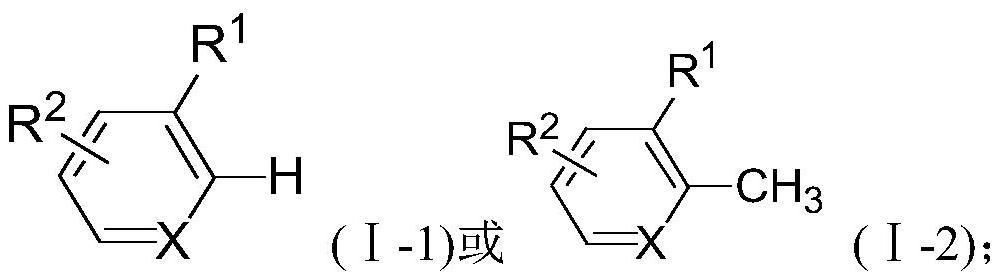
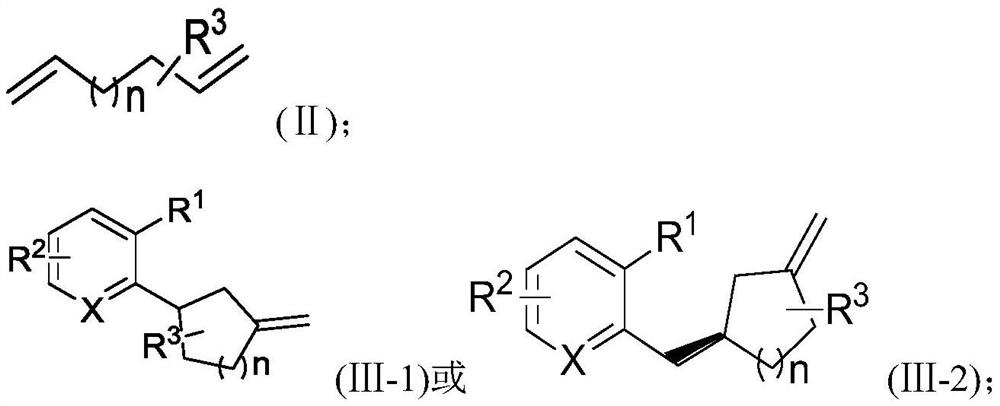
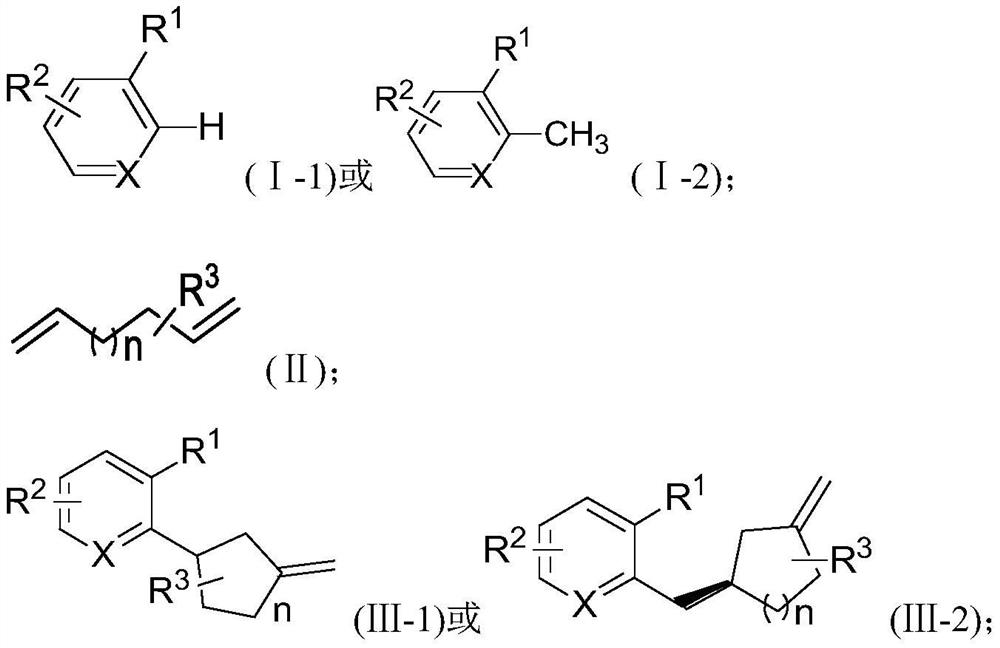
[0031] A method for synthesizing carbocyclic derivatives substituted by unsaturated double bonds, comprising the steps of:
[0032] Under the protection of an inert gas, in an organic solvent, the compound of the formula (I-1) reacts with the compound of the formula (II) under the action of a catalyst and a cocatalyst to obtain a carbocyclic ring substituted with a double bond having the structure of the compound of the formula (III-1) Compounds;
[0033] Or, under the protection of an inert gas, in an organic solvent, the compound of the formula (I-2) reacts with the compound of the formula (II) under the action of a catalyst and a cocatalyst to obtain a compound having a structure of the formula (III-2) substituted with a double bond Carbocyclic compounds;
[0034] The catalyst is an organic rare earth compound; the cocatalyst is a boron salt;
[0035]
[0036] in,
[0037] X is C or N;
[0038] When X is N, R 1 , R 2 each independently selected from a hydrogen atom...
Embodiment 1
[0073] A method for synthesizing carbocyclic derivatives substituted by unsaturated double bonds, comprising the steps of:
[0074]
[0075] Under nitrogen atmosphere, the cocatalyst [Ph 3 C][B(C 6 f 5 ) 4 ] (18.5mg, 0.02mmol) was dissolved in chlorobenzene (1mL) to obtain a cocatalyst solution, and the catalyst (η 5 -C 5 Me 4 -C 5 h 3 NC 6 h 5 )Sc(CH 2 SiMe 3 ) 2 (THF) (10.1 mg, 0.02 mmol) was dissolved in chlorobenzene (1 mL) to obtain a catalyst solution. Then 2-methylanisole 1a (122.2mg, 1.0mmol) and 1,5-hexadiene 2a (205mg, 2.5mmol) were added to the reaction system, stirred magnetically at 70°C for 4h, and analyzed by thin layer chromatography ( TLC) to monitor the progress of the reaction. After the reaction, using n-hexane as the mobile phase, separation and purification by column chromatography gave the corresponding catalytic product, a colorless liquid 3a, with a yield of 71%.
[0076] NMR characterization is as follows: 1 H NMR (400MHz, CDCl 3 )...
Embodiment 2
[0078] A method for synthesizing carbocyclic derivatives substituted by unsaturated double bonds, comprising the steps of:
[0079]
[0080] Under nitrogen atmosphere, the cocatalyst [Ph 3 C][B(C 6 f 5 ) 4 ] (18.5mg, 0.02mmol) was dissolved in chlorobenzene (1mL) to obtain a cocatalyst solution, and the catalyst ((η 5 -C 5 Me 4 -C 5 h 3 NBr)Sc(CH 2 SiMe 3 ) 2 (THF) (11.2mg, 0.02mmol) was dissolved in chlorobenzene (1mL) solution to obtain a catalyst solution, the cocatalyst solution was slowly added dropwise to the stirred catalyst solution, and then 2,3-benzofuran 1b (1.0mmol ) and 1,5-hexadiene 2a (123mg, 1.5mmol) were added to the reaction system, magnetically stirred at 60°C for 4h, and the reaction progress was monitored by TLC. After the reaction, using n-hexane as the mobile phase, separation and purification by column chromatography gave the corresponding catalytic product, a colorless liquid 3ba, with a yield of 64%.
[0081] NMR characterization is as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com