Synthetic method for derivatization of alpha-C (sp3)-H bond of cyclopropane compound
A synthesis method and compound technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of complex reaction steps, unfriendly environment, harsh synthesis reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
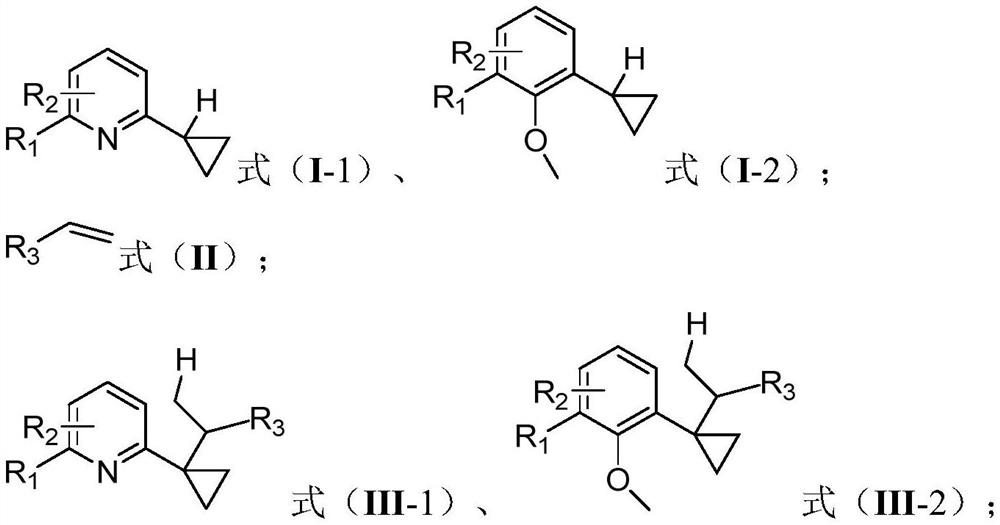
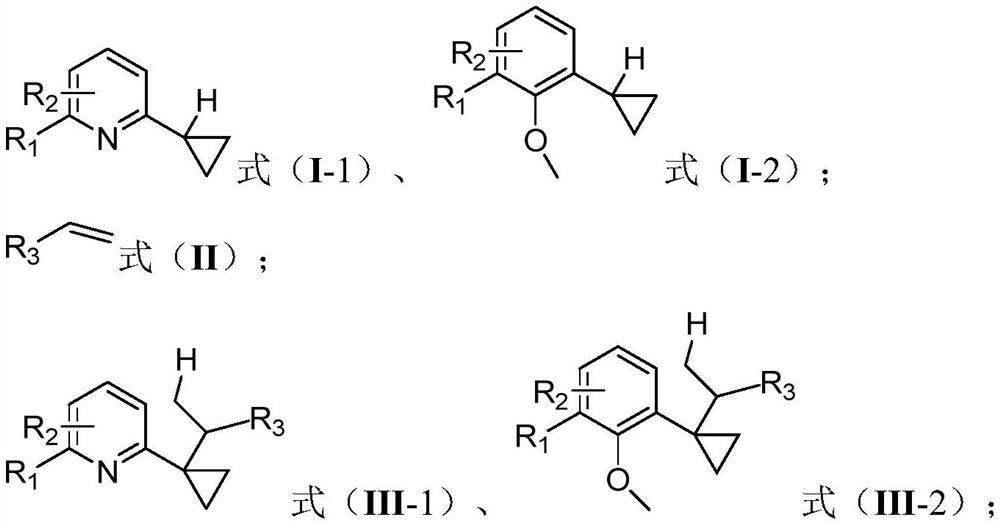
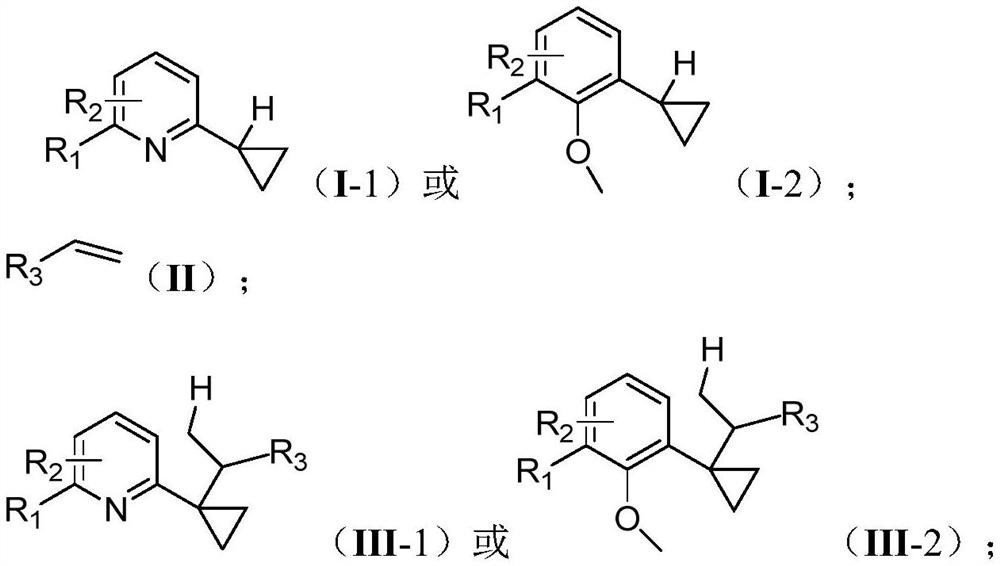
[0029] One embodiment of the present invention proposes a cyclopropane compound α-C(sp 3 )-H bond derivatized synthetic method, comprising the steps:
[0030] Under the protection of an inert gas, in an organic solvent, the compound having the structure shown in the formula (I-1) reacts with the compound of the formula (II) under the action of a catalyst and a cocatalyst to obtain a compound having the structure of the compound of the formula (III-1) ;
[0031] Or, under the protection of an inert gas, in an organic solvent, the compound having the structure shown in formula (I-2) reacts with the compound of formula (II) under the action of catalyst and cocatalyst to obtain the compound structure of formula (III-2) compound of;
[0032] The catalyst is an organic rare earth compound; the cocatalyst is a boron salt;
[0033]
[0034] Among them, R 2 for R 1 The ortho-substitution, meta-substitution or para-substitution;
[0035] R 1 , R 2 independently selected from ...
Embodiment 1
[0056] A cyclopropane compound α-C(sp 3 )-H bond derivatized synthetic method, comprising the steps:
[0057] Under nitrogen atmosphere, the catalyst (η 2 -N-P-Ph 2 )Ln(CH 2 C 6 h 4 NMe 2 -o) 2 (28.8mg, 0.04mmol) was dissolved in 1mL chlorobenzene solvent and was added to a 50mL storage bottle for continuous stirring, and the promotor [Ph 3 C][B(C 6 f 5 ) 4 ] (36.9mg, 0.04mmol) was dissolved in 1mL of chlorobenzene solvent and slowly added dropwise to the chlorobenzene solution of the catalyst, then 2-cyclopropyl-6 ethyl-pyridine (147mg, 1.0mmol), 1-octene (448mg, 4.0mmol) was added into the reaction system, and a 50mL storage bottle was placed in a parallel reactor at 100°C to stir the reaction. The reaction was monitored by TLC. After the reaction was completed, it was cooled to room temperature, and the pure product was separated through a silica gel chromatography column (the mobile phase was 2% ethyl acetate in hexane), and the yield was 93%.
[0058] NMR ch...
Embodiment 2
[0060] A cyclopropane compound α-C(sp 3 )-H bond derivatized synthetic method, comprising the steps:
[0061] Under nitrogen atmosphere, the catalyst (η 2 -N-P-Ph 2 )Ln(CH 2 C 6 h 4 NMe 2 -o) 2 (28.8mg, 0.04mmol) was dissolved in 1mL chlorobenzene solvent and was added to a 50mL storage bottle for continuous stirring, and the promotor [Ph 3 C][B(C 6 f 5 ) 4 ] (36.9mg, 0.04mmol) was dissolved in 1mL of chlorobenzene solvent and slowly added dropwise to the chlorobenzene solution of the catalyst, then 2-cyclopropyl-6-isopropyl-pyridine ((161mg, 1.0mmol), 1 -Octene (448mg, 4.0mmol) joins in the reaction system, and 50mL liquid storage bottle is placed in the parallel reactor of 100 ℃ and stirs reaction.Reaction is monitored with TLC, is cooled to room temperature after completion of reaction, passes through silica gel chromatographic column (flow 2% ethyl acetate in hexane) to isolate the pure product with a yield of 91%.
[0062] NMR characterization is as follows:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com