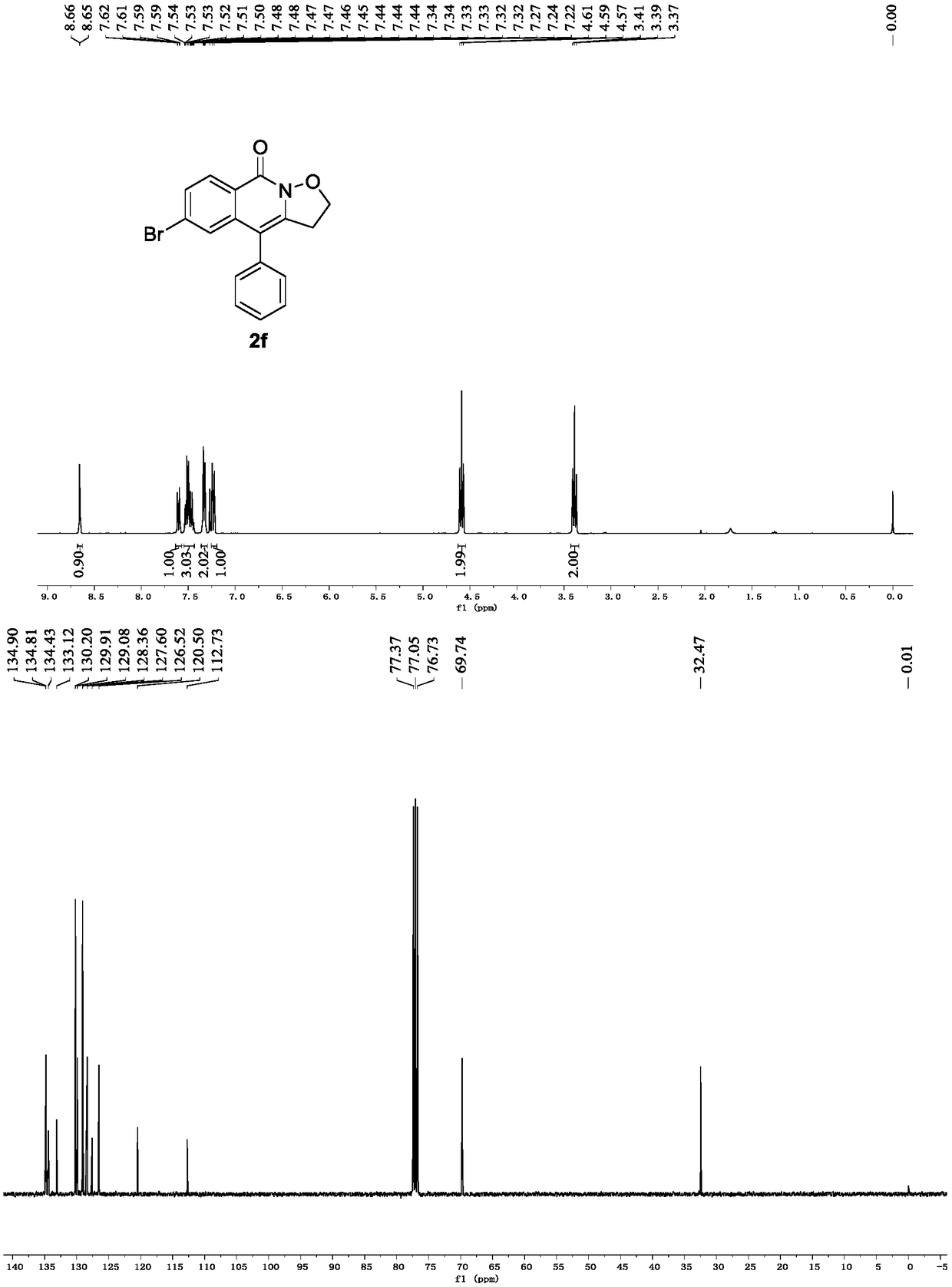
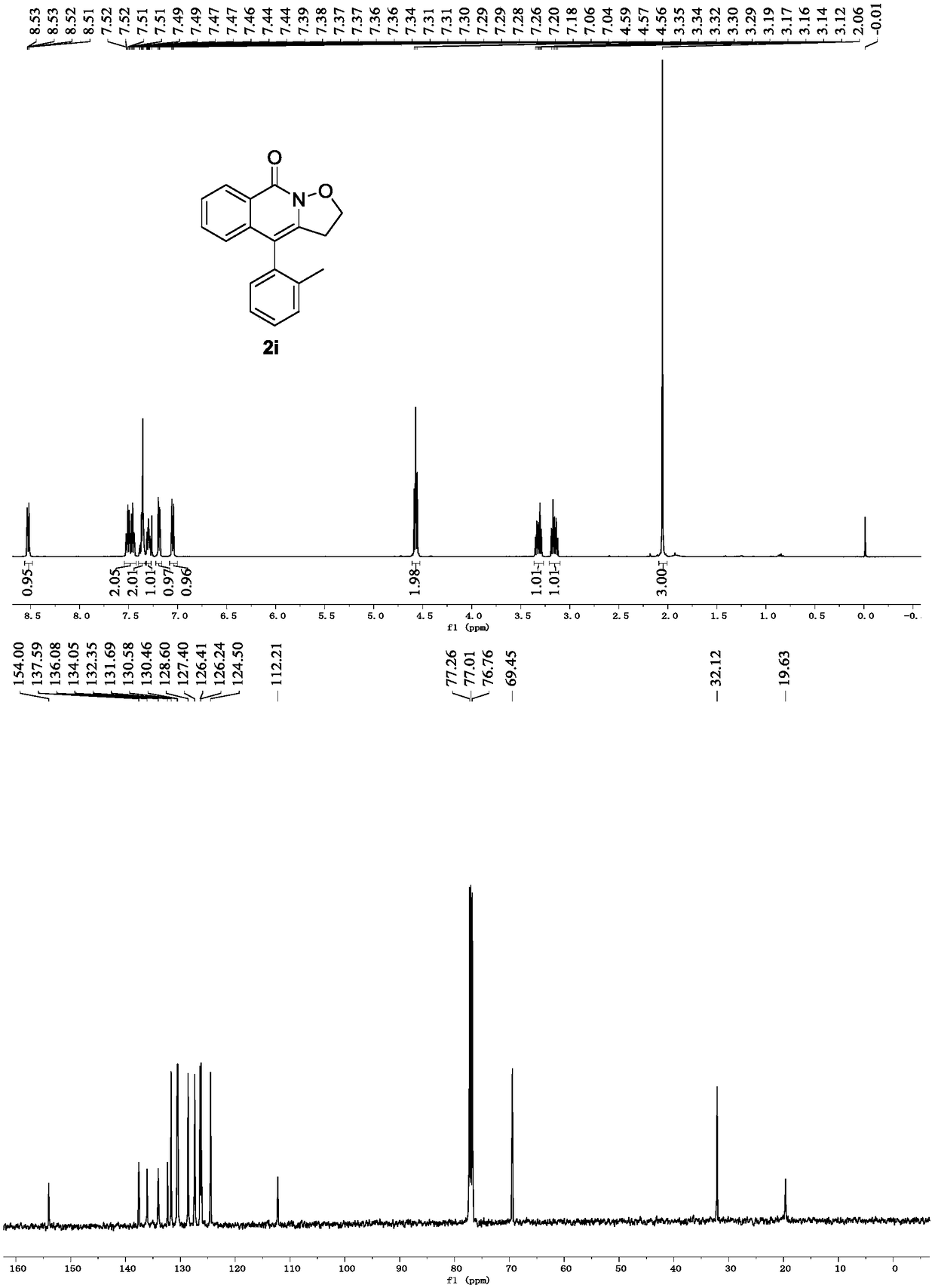
Preparation method of isoxazole and isoquinoline ketone derivative
A technology of isoquinolinone and isoxazole, which is applied in the field of preparation of isoxazoloisoquinolinone derivatives, can solve the problems of high reaction temperature and long reaction time, and achieve simple synthesis method, easy product, and easy reaction fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Preparation of isoxazoloisoquinolinone derivative 2a
[0022]
[0023] Add N-alkoxybenzamide 1a (0.2mmol, 53.0mg), iodobenzene diacetate (0.2mmol, 64.4mg) into a 25ml round bottom flask, add hexafluoroisopropanol (4mL), stir at room temperature, React for 1 minute. After the reaction is complete, use a rotary evaporator to remove the solvent to obtain a crude product, which is separated by column chromatography (200-300 mesh silica gel) (petroleum ether / ethyl acetate=1 / 1), and uses a rotary evaporator to remove the solvent to obtain the target The product is the unsubstituted isoxazoloisoquinolinone derivative 2a, and its yield is 92%.
[0024] Spectral analysis data 2a:
[0025] 1 H NMR (400MHz, CDCl 3 )δ8.54(d, J=7.1Hz, 1H), 7.50(tq, J=14.5, 7.2Hz, 5H), 7.36(t, J=7.7Hz, 3H), 4.58(t, J=7.6Hz, 2H), 3.39(t, J=7.6Hz, 2H). 13 C NMR (100MHz, CDCl 3 )δ153.95, 136.20, 135.02, 132.52, 131.63, 130.31, 128.93, 128.10, 127.47, 126.32, 124.71, 113.03, 69.55, 32.46.
Embodiment 2
[0027] Replace 1a in Example 1 with 1b, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0028]
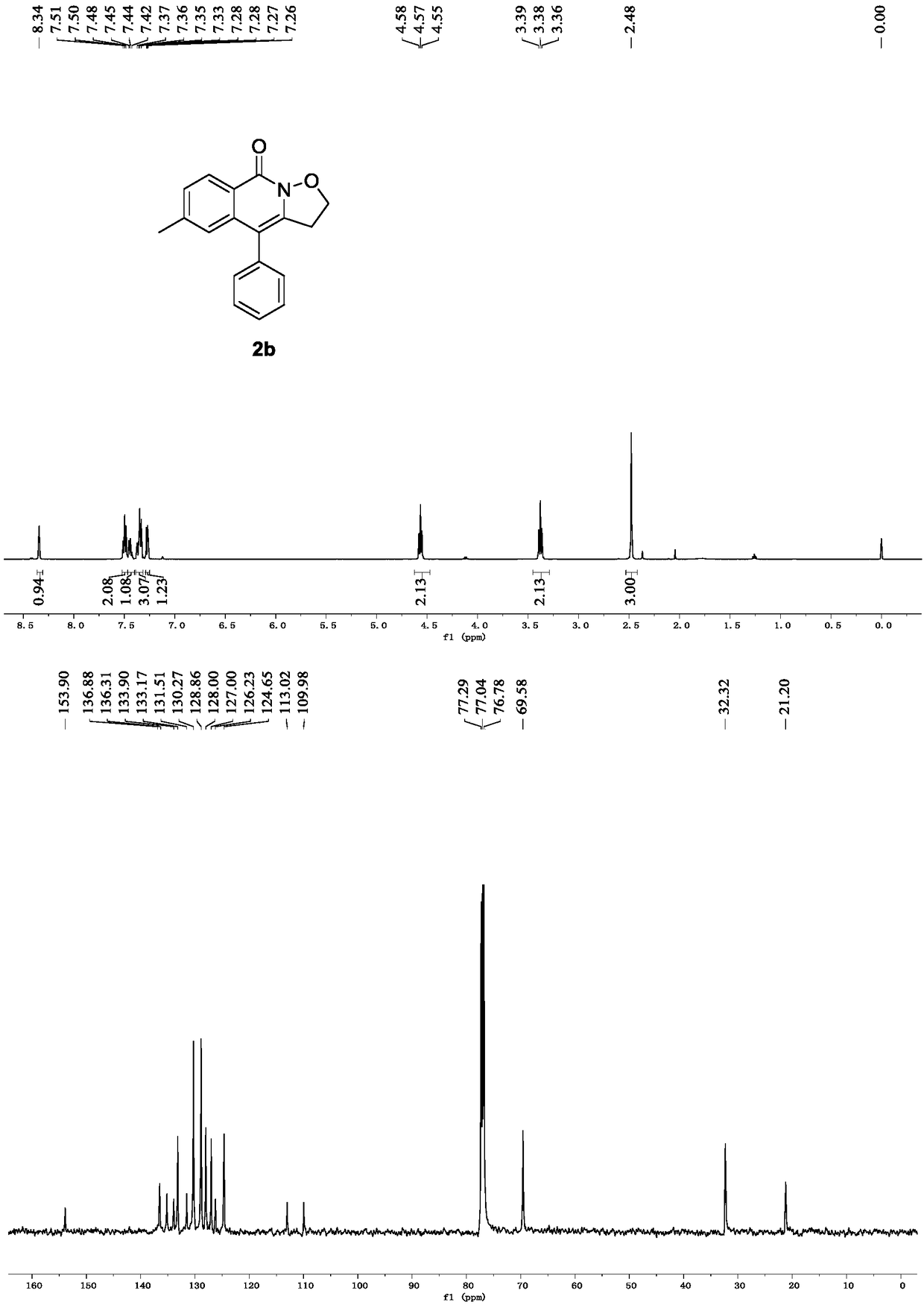
[0029] Spectrum analysis data 2b:
[0030] 1 H NMR (500MHz, CDCl 3 )δ8.34(s, 1H), 7.50(t, J=7.5Hz, 2H), 7.44(t, J=6.9Hz, 1H), 7.35(dd, J=12.4, 8.1Hz, 3H), 7.27( dd, J=5.9, 2.3Hz, 1H), 4.57(t, J=7.5Hz, 2H), 3.38(t, J=7.6Hz, 2H), 2.48(s, 3H). 13 C NMR (125MHz, CDCl 3 )δ153.90, 136.88, 133.90, 133.17, 131.51, 130.27, 128.86, 128.00, 127.00, 126.23, 124.65, 113.02, 109.98, 69.58, 32.32, 21.20.
Embodiment 3
[0032] Replace 1a in Example 1 with 1c, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0033]
[0034] Spectrum analysis data 2c:
[0035] 1 H NMR (500MHz, CDCl 3 )δ7.90(d, J=2.7Hz, 1H), 7.47(t, J=7.3Hz, 2H), 7.42(t, J=7.4Hz, 1H), 7.35–7.30(m, 2H), 7.30– 7.25(m,1H),7.13(dd,J=9.0,2.7Hz,1H),4.56(t,J=7.5Hz,2H),3.91(s,3H),3.36(t,J=7.6Hz,2H ). 13 C NMR (125MHz, CDCl 3 )δ158.33, 153.42, 135.12, 130.19, 128.81, 127.97, 126.27, 122.21, 113.01, 107.13, 69.70, 55.71, 32.13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com