A method for removing chlorine from zinc sulfate solution
A zinc sulfate solution and solution technology, applied in the field of chlorine removal, can solve the problems of reduced dechlorination efficiency, low dechlorination efficiency, zinc loss, etc., and achieve the effect of improving chlorine removal efficiency, reducing chlorine removal cost, and improving removal rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
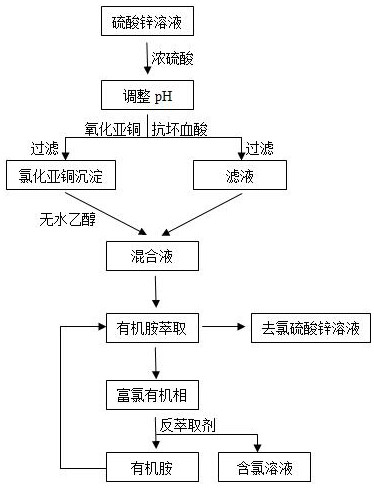
[0026] A kind of method for removing chlorine from zinc sulfate solution, its steps are: get 500mL zinc sulfate solution, add 10mol / L concentrated sulfuric acid to adjust pH, make solution pH be 3, wherein chloride ion concentration is 1g / L; Add 5 % ascorbic acid, then add 3g of copper sulfate, react for 40min, obtain the cuprous chloride precipitate and filter it; rinse the cuprous chloride precipitate with absolute ethanol, combine the washing solution and the filtrate to obtain a mixed solution; then add an equal volume of Chlorine in the mixed liquid is extracted by the extract after mixing dimethylamine and trimethylamine, wherein the extract is made of dimethylamine and trimethylamine at a volume ratio of 1:1, after extraction for 4 hours, stand and separate to obtain Chlorine-removing zinc sulfate solution and chlorine-rich organic phase; adding 2% sodium sulfate solution to the chlorine-rich organic phase to extract the chlorine in the chlorine-rich organic phase. Duri...
Embodiment 2
[0029] A kind of method for removing chlorine from zinc sulfate solution, its steps are: get 500mL zinc sulfate solution, add 20mol / L concentrated sulfuric acid to adjust pH, make solution pH be 1, wherein chloride ion concentration is 1g / L; Add 10 % ascorbic acid, then add 3g of copper sulfate, react for 60min, obtain the cuprous chloride precipitate and filter it; rinse the cuprous chloride precipitate with absolute ethanol, combine the washing liquid and the filtrate to obtain a mixed solution; then add an equal volume of Chlorine in the mixed liquid is extracted by the extract after mixing dimethylamine and n-butylamine, wherein the extract is made of dimethylamine and trimethylamine at a volume ratio of 1:1, after extraction for 6 hours, stand and separate to obtain Chlorine-removed zinc sulfate solution and chlorine-rich organic phase; add 5% sodium nitrate solution to the chlorine-rich organic phase to extract the chlorine in the chlorine-rich organic phase, the strippin...
Embodiment 3
[0032] A method for removing chlorine from zinc sulfate solution, the steps are: get 500mL zinc sulfate solution and add 15mol / L concentrated sulfuric acid to adjust the pH, so that the pH of the solution is 2, and wherein the chloride ion concentration is 1g / L; % ascorbic acid, then add 3g of copper sulfate, react for 50min, obtain the cuprous chloride precipitate and filter it; wash the cuprous chloride precipitate with absolute ethanol, combine the washing solution and the filtrate to obtain the mixed solution; then add an equal volume of Chlorine in the mixed liquid is extracted by the mixed extract of trimethylamine and n-butylamine, wherein the extract is made of dimethylamine and trimethylamine at a volume ratio of 1:1. After extraction for 5 hours, it is allowed to stand for stratification to remove Chlorine zinc sulfate solution and chlorine-rich organic phase; add 6% sodium nitrate solution to the chlorine-rich organic phase to strip the chlorine in the chlorine-rich ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com