Transdermal absorption composition and application of same to preparation of transdermal absorption preparation
A composition and preparation technology, which is applied in the field of transdermal drug delivery preparations, can solve the problems of limited permeation-promoting effect, achieve the effects of reducing toxic side effects and skin irritation, prolonging release, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Pre-treatment of pigskin: take fresh pigskin, remove the subcutaneous fat layer, shave the pig hair to a length no longer than 2mm, and use it immediately or freeze it at -20°C to -80°C for later use.
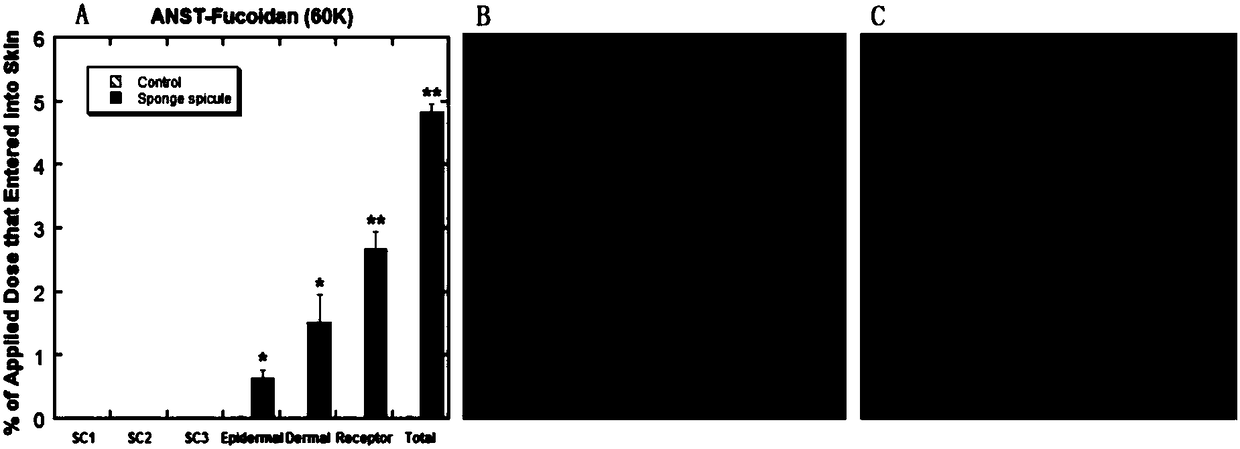
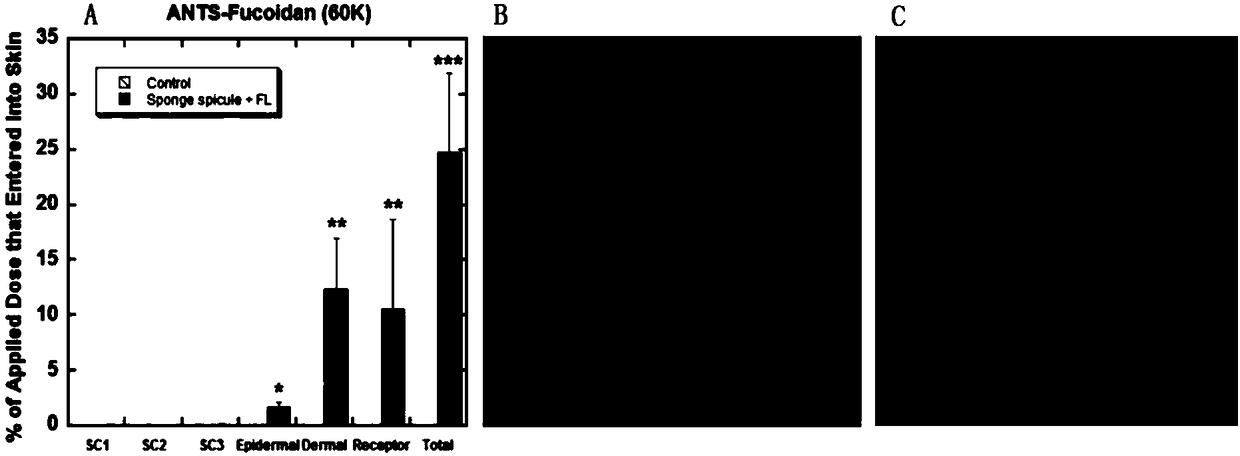
[0045] (2) Preparation of flexible nanoliposomes: phospholipid 90G (100mg / ml), surfactant polyoxyethylene (20) oleyl ether (100mg / ml) and methanol / chloroform=1:1 were added to the In a round flask, it was evaporated to dryness by a rotary evaporator to form a uniform film on the bottom surface of the round flask. Then add 1 to 5 ml of ANTS-Fucoidan (fucoidan) drug solution, and perform hydration by shaking / ultrasound and other steps. Use the imported special extrusion device for liposomes, squeeze 21 times, and finally transfer to a new EP tube to obtain ANTS-Fucoidan flexible nano-liposome solution of 1-100 mg / ml, ready for use.
[0046] (3) Preparation of sponge spicule solution: add a certain amount of PBS phosphate buffer saline, deionized water, normal saline o...
Embodiment 2
[0052] (1) Pretreatment of pigskin: with embodiment 1.
[0053] (2) Preparation of ordinary nanoliposomes: add phospholipid 90G (100mg / ml) and methanol / chloroform=1:1 into a round flask in a certain ratio, evaporate to dryness by a rotary evaporator, and make it in a round shape Form a uniform film on the bottom surface of the flask; then add 1 to 5ml of FITC-Hyaluronic acid (hyaluronic acid) drug solution, hydrate through oscillation / ultrasound and other steps, use imported liposome special extrusion device, and squeeze 21 times , and finally transferred to a new EP tube to obtain a 1-100 mg / ml FITC-Hyaluronic acid common nanoliposome solution for use.
[0054] (3) Preparation of flexible nanoliposomes: similar to Example 1.
[0055] (4) Preparation of sponge spicule solution: same as Example 1.
[0056] (5) In vitro skin penetration test: similar to Example 1. Add 150 μL of FITC-Hyaluronic acid (average molecular weight 250KDa) solution containing 1-100mg / ml or FITC-Hyalu...
Embodiment 3
[0064] (1) Pretreatment of pigskin: with embodiment 1.
[0065] (2) Preparation of solid lipid nanoparticles: prepared with reference to conventional techniques in the art.
[0066] (3) Preparation of sponge spicule solution: same as in Example 1.
[0067] (4) In vitro transdermal test: carried out with reference to Example 1. The results showed that the spongy spicules combined with solid lipid nanoparticles had obvious penetration-promoting effect compared with other groups.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com