Imipenem and cilastatin sodium for injection and preparation process of imipenem and cilastatin sodium for injection
A technology for imipenem cilastatin sodium and cilastatin sodium, which is applied in the field of imipenem cilastatin sodium for injection and its production technology, can solve the problems of different quality, great difficulty in mixing, and powder particles. The problem of huge difference in diameter and specific gravity can reduce the influence of particle dust, which is beneficial to uniformity and is not easy to delaminate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
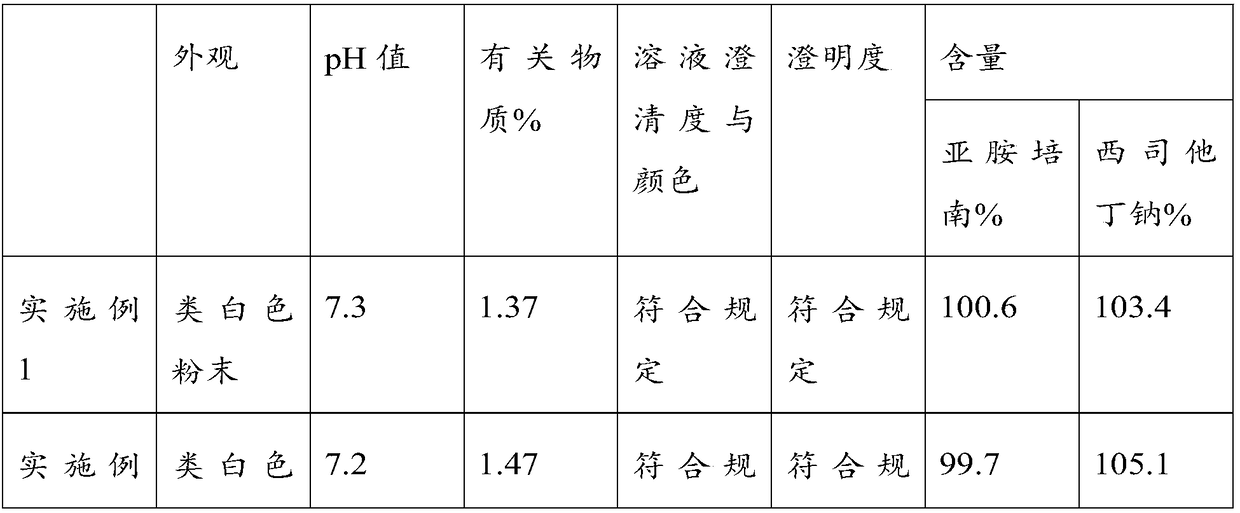
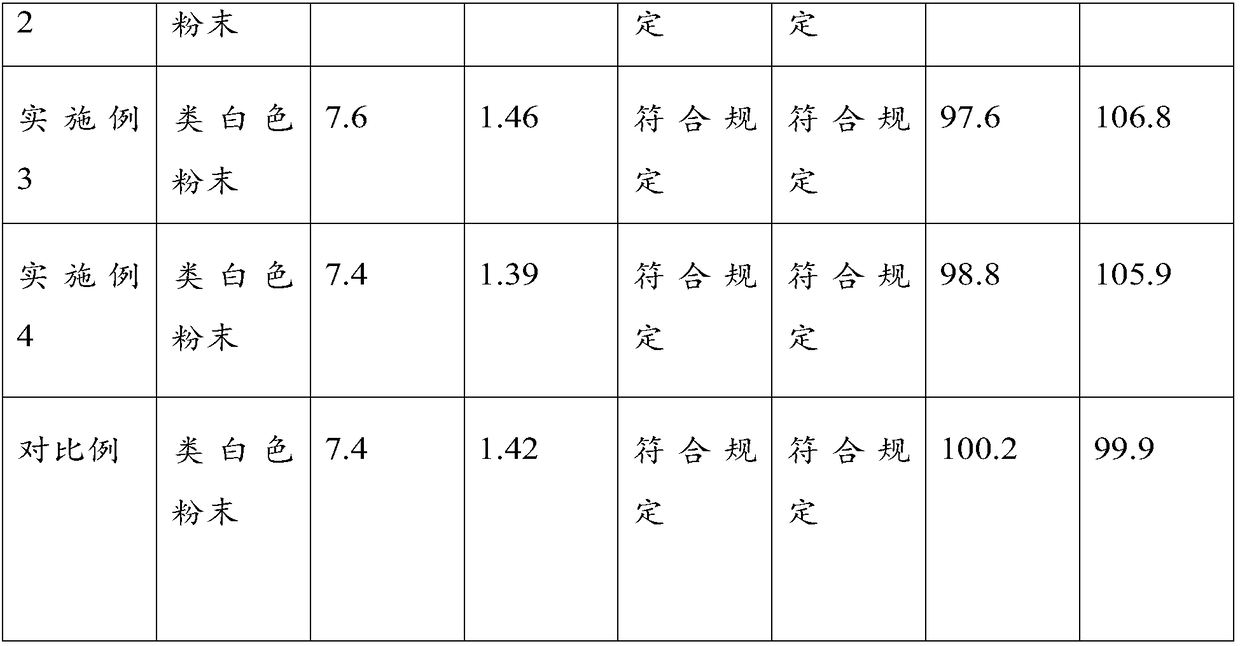
Embodiment 1
[0030] A kind of imipenem cilastatin sodium for injection, it is made by following production process:
[0031] Mix imipenem, cilastatin sodium and sodium bicarbonate with a mass ratio of 1:1:0.04 in sequence in a sterile room with a humidity less than 40%, and then feed them by screw under the condition that the humidity is less than 40%. The lower portion is packed in sterile glass bottles, and the sterile glass bottle caps are covered with sterilized and dried rubber stoppers and sterilized and dried aluminum caps, and the caps are crimped and sealed.
[0032] Among them, the particle size of imipenem is controlled at 68.8-80.7 microns, that of cilastatin sodium is controlled at 270-320 microns, and that of sodium bicarbonate is controlled at 110-120 microns.
Embodiment 2
[0034] A kind of imipenem cilastatin sodium for injection, it is made by following production process:
[0035] Mix imipenem, cilastatin sodium and sodium bicarbonate with a mass ratio of 1:1:0.04 in sequence in a sterile room with a humidity less than 40%, and then feed them by screw under the condition that the humidity is less than 40%. The lower portion is packed in sterile glass bottles, and the sterile glass bottle caps are covered with sterilized and dried rubber stoppers and sterilized and dried aluminum caps, and the caps are crimped and sealed.
[0036] Among them, the particle size of imipenem is controlled at 68.8-70 microns, that of cilastatin sodium is controlled at 300-320 microns, and that of sodium bicarbonate is controlled at 115-120 microns.
Embodiment 3
[0038] A kind of imipenem cilastatin sodium for injection, it is made by following production process:
[0039] Mix imipenem, cilastatin sodium and sodium bicarbonate with a mass ratio of 1:1:0.04 in sequence in a sterile room with a humidity less than 40%, and then feed them by screw under the condition that the humidity is less than 40%. The lower portion is packed in sterile glass bottles, and the sterile glass bottle caps are covered with sterilized and dried rubber stoppers and sterilized and dried aluminum caps, and the caps are crimped and sealed.
[0040] Among them, the particle size of imipenem is controlled at 76-80.7 microns, that of cilastatin sodium is controlled at 270-280 microns, and that of sodium bicarbonate is controlled at 110-120 microns.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com