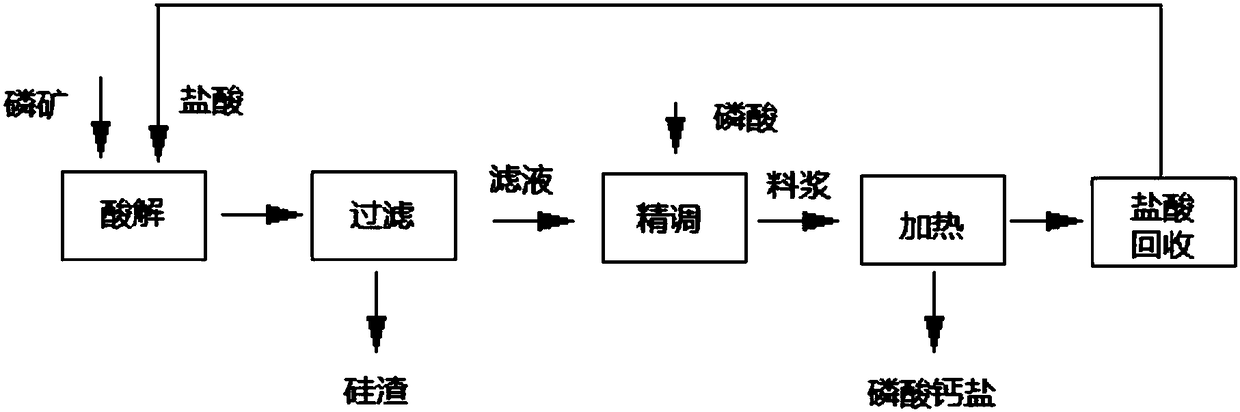
Method for producing calcium phosphate salts from hydrochloric acid and phosphate rock
A technology of calcium phosphate and calcium dihydrogen phosphate, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as affecting sustainable development, waste of resources, etc., to promote product production, save costs, and reduce prices. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The main components of the phosphate rock used in this embodiment are shown in the following table 1, and the main components of the phosphoric acid adopted are shown in the following table 2:
[0056] Table 1
[0057] raw material name
P 2 o 5 %
MgO%
CaO%
Fe 2 o 3 %
al 2 o 3 %
Medium grade phosphate rock
26.32
1.78
40.98
1.73
1.53
[0058] Table 2
[0059] raw material name
P 2 o 5 %
CaO%
MgO%
Fe 2 o 3 %
Al 2 o 3 %
concentrated phosphoric acid
54.88
not detected
2.35
0.30
0.20
[0060] A. Mix medium-grade phosphate rock with 21% hydrochloric acid at a molar ratio of CaO:HCl=1:2.1, then weigh 5000g of medium-grade ore, 13221g of 21% hydrochloric acid, and carry out acid hydrolysis for 1 hour at 35°C. The slurry is filtered and washed three times with 1500g of clear water to obtain the filtrate and filter residue; after testing, the m...
Embodiment 2
[0067] The main components of the phosphate rock adopted in this embodiment are shown in the following table 4, and the main components of the phosphoric acid adopted are shown in the following table 5:
[0068] Table 4
[0069] raw material name
P 2 o 5 %
MgO%
CaO%
Fe 2 o 3 %
Al 2 o 3 %
Phosphate concentrate
31.96
0.62
46.42
0.88
0.81
[0070] table 5
[0071] raw material name
P 2 o 5 %
CaO%
MgO%
Fe 2 o 3 %
al 2 o 3 %
wet process phosphoric acid
42.16
0.10
2.09
0.44
0.37
[0072] A. Mix phosphor concentrate and hydrochloric acid with a mass concentration of 30% at a molar ratio of CaO:HCl=1:1.8, then weigh 5000g of phosphor concentrate, 9077g of 30% hydrochloric acid, and carry out acid hydrolysis reaction at 60°C for 0.5h. The slurry is filtered and washed three times with 1500g of clear water to obtain the filtrate and filter residue; af...
Embodiment 3
[0079] The main components of the phosphate rock used in this embodiment are shown in the following table 7, and the main components of the phosphoric acid used are shown in the following table 8:
[0080] Table 7
[0081] raw material name
P 2 o 5 %
MgO%
CaO%
Fe 2 o 3 %
Al 2 o 3 %
Medium grade phosphate rock
26.32
1.78
40.98
1.73
1.53
[0082] Table 8
[0083] raw material name
P 2 o 5 %
CaO%
MgO%
Fe 2 o 3 %
al 2 o 3 %
wet process phosphoric acid
42.16
0.10
2.09
0.44
0.37
[0084] A. Mix medium-grade phosphate rock with 25% hydrochloric acid at a molar ratio of CaO:HCl=1:2.3, then weigh 500g of medium-grade phosphate rock, 1216g of 25% hydrochloric acid, and carry out acid hydrolysis reaction at 40°C for 0.5 h, the slurry is filtered and washed three times with 150g of clear water to obtain filtrate and filter residue; after testing, the ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com