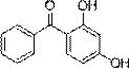
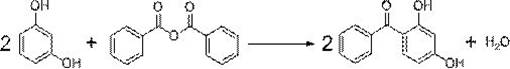
A kind of green synthesis technology of 2,4-dihydroxybenzophenone
A technology of dihydroxybenzophenone and synthesis process, which is applied in the direction of condensation preparation of carbonyl compounds, physical/chemical process catalysts, organic chemistry, etc., and can solve the problems of HCl acid mist, waste water and exhaust gas discharge, equipment corrosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
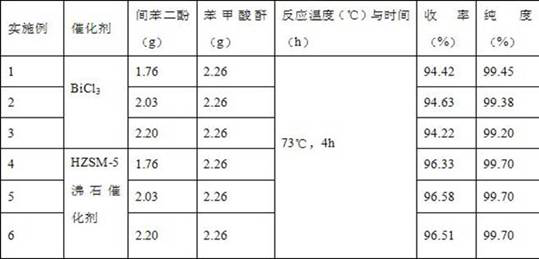
[0037] Weigh 1.76g resorcinol, 2.26g benzoic anhydride, 0.16gBiCl 3 . Dissolve resorcinol in ethanol at room temperature to form 60°C saturated solution a, and dissolve benzoic anhydride in ethanol to form 60°C saturated solution b. BiCl 3 Put the saturated solution a in the reactor, stir and add the saturated solution b dropwise to it, and fully react at 73°C for 4 hours. After the reaction, cool to 5°C, filter, recrystallize and refine the filter residue with an organic solvent of ethanol:water=4:1 (volume ratio, the same below), weigh 3.23g after drying, and detect 2,4- The content of dihydroxybenzophenone is 99.45%, and the yield is 94.42%.
Embodiment 2
[0039] Weigh 2.03g resorcinol, 2.26g benzoic anhydride, 0.17gBiCl 3 . Dissolve resorcinol in ethanol at room temperature to form 60°C saturated solution a, and dissolve benzoic anhydride in ethanol to form 60°C saturated solution b. BiCl 3 and saturated solution a are placed in a reactor, stirred, and saturated solution b is added dropwise thereinto, and fully reacted at 73° C. for 4 hours. After the reaction, cool to 5°C, filter, recrystallize and refine the filter residue with an organic solvent of ethanol:water=4:1, then dry it and weigh 3.75g, and detect 2,4-dihydroxybenzophenone by HPLC The content of ketone is 99.38%, and the yield is 94.63%.
Embodiment 3
[0041] Weigh 2.20g resorcinol, 2.260g benzoic anhydride, 0.22gBiCl 3 . Dissolve resorcinol in ethanol at 60°C to form a saturated solution a, and dissolve benzoic anhydride in ethanol to form a 60°C saturated solution b. BiCl 3 Put the saturated solution a in the reactor, stir and add the saturated solution b dropwise to it, and fully react at 73°C for 4 hours. After the reaction, cool to 5°C, filter, recrystallize and refine the filter residue with an organic solvent of ethanol:water=4:1, weigh 4.03g after drying, and detect 2,4-dihydroxybenzophenone by HPLC The content of ketone is 99.20%, and the yield is 94.22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com