Application of N-(thiazole-2-yl)-3-(piperazine-1-yl)propionamide compound in preparation of medicines
A compound, propionamide technology, applied in the field of medicine, can solve problems such as no treatment means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
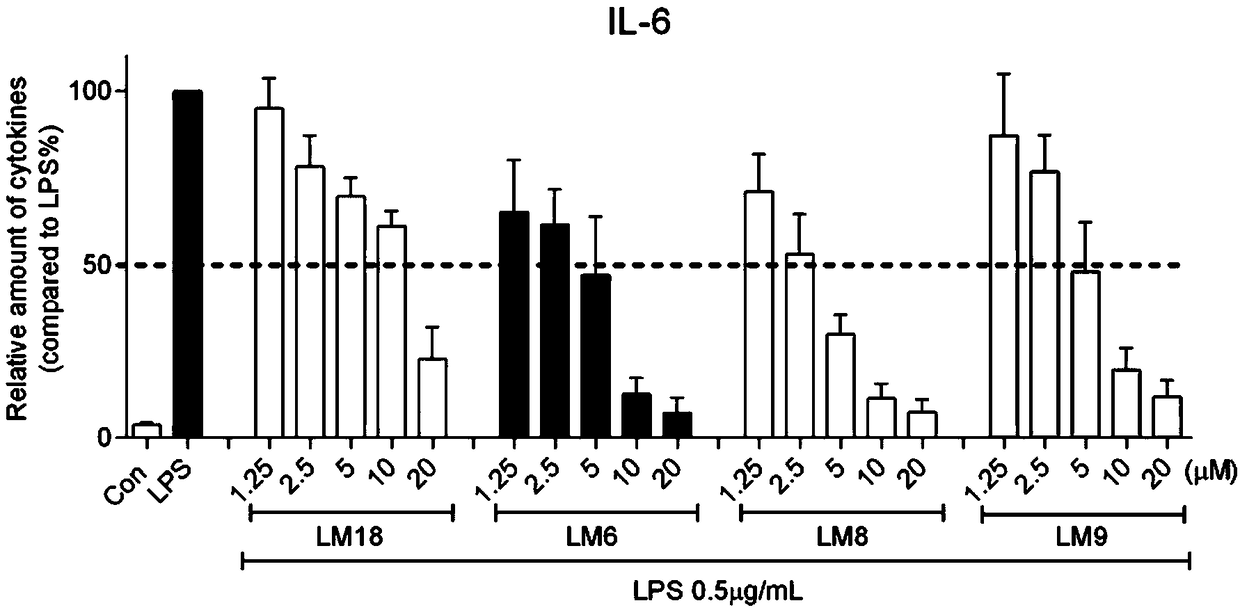
[0026] Example 1 The dose-effect relationship of the active compound inhibiting the release of inflammatory factors from macrophages stimulated by LPS
[0027] In order to test the dose-effect relationship of active compounds inhibiting the release of IL-6 from RAW 264.7 macrophages stimulated by LPS. The specific method is as follows: 1.2×10 6 Primary macrophages were cultured with DMEM medium at 37°C. After 24 hours, the medium was renewed, and the test compound (final concentration: 10 μM) was added for pretreatment for 2 hours, and then treated with 0.5 μg / mL LPS for 22 hours. , collect the culture fluid and use the ELISA method to detect the IL-6 content; collect the cells to detect the total protein concentration, and the ELISA results are divided by the corresponding total protein concentration for calibration. The IL-6 content of the LPS control group is calibrated as 100, and the average value is calculated and error value. See the experimental results figure 1 . ...
Embodiment 2
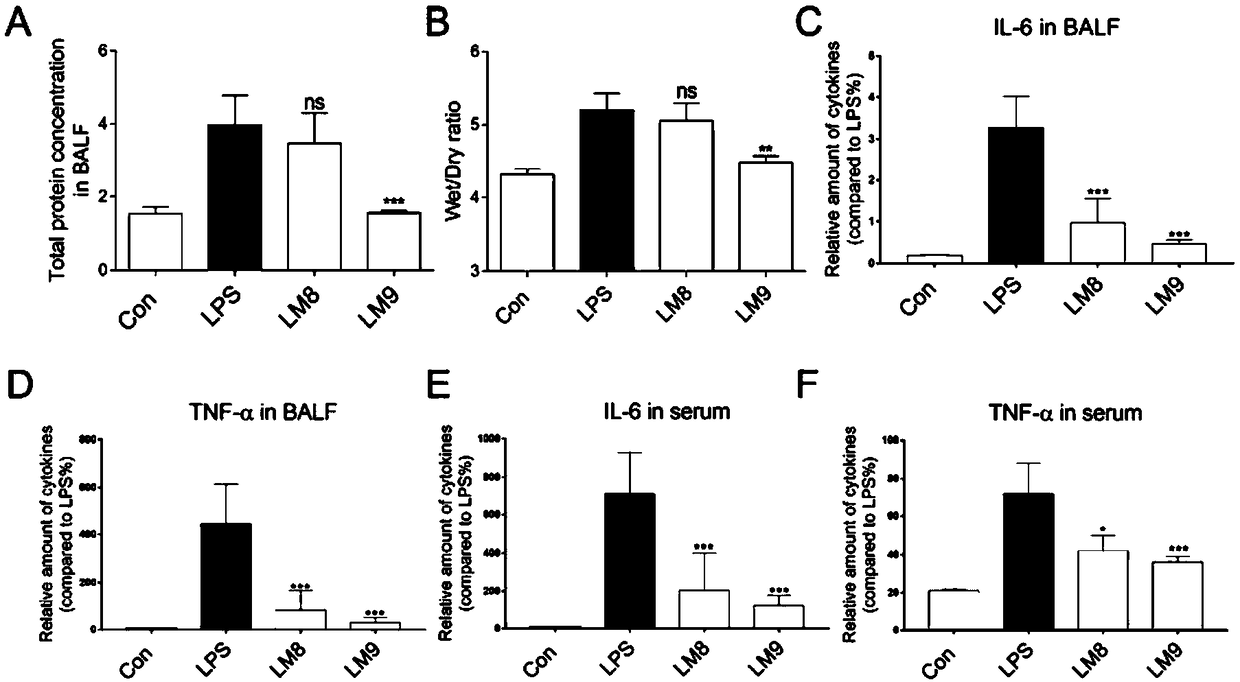
[0028] Compound of Example 2 alleviates physiological changes in rats with acute lung injury
[0029] Suspensions were prepared with 0.5% sodium carboxymethylcellulose and compounds LM8 and LM9 for intraperitoneal administration. Rats in each group were exposed to the trachea after ether anesthesia. Except for the control group, 50 μL 5 mg / kg LPS was slowly instilled into the trachea of the other groups to cause acute lung injury in the rats. Wounds were used to establish an acute lung injury model. After 24 hours of animal modeling, mice were anesthetized by intraperitoneal injection of 10% chloral hydrate at a dose of 5 mL / kg, the left lung was ligated by thoracotomy, and bronchoalveolar lavage was performed on the right lung with 1 mL of normal saline, and the lavage fluid was collected. The same operation was repeated for 3 Second-rate.
[0030] After the alveolar lavage fluid was collected, it was centrifuged at 1000 rpm at 4°C for 5 minutes, and the supernatant was t...
Embodiment 3
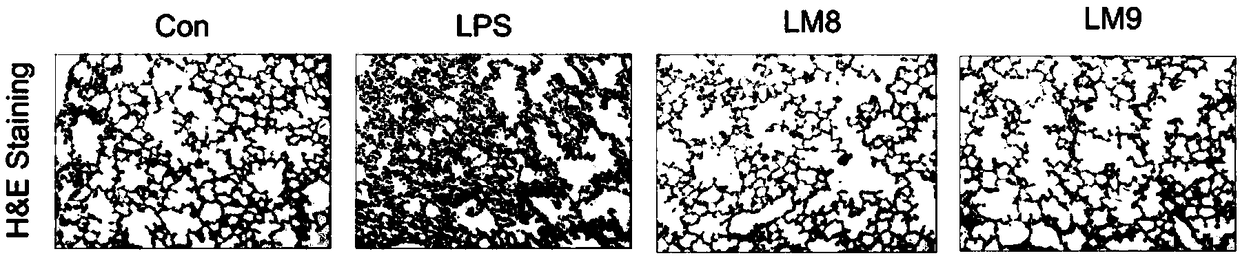
[0032] Example 3 compound alleviates the pathological change test of lung tissue in acute lung injury
[0033] For experimental data, see image 3 , the alveolar cavity of rats in the normal control group was clear, the structure was complete, and the wall was smooth; after the acute lung injury model was induced by tracheal instillation of LPS, the alveolar wall was obviously edematized and thickened, and the infiltration of inflammatory cells increased; after treatment with compounds LM8 and LM9, the cells edema, The thickening was significantly weakened, and the infiltration of inflammatory cells was significantly reduced, which was not much different from the normal group, indicating that the compound can effectively relieve lung tissue damage in acute lung injury.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com