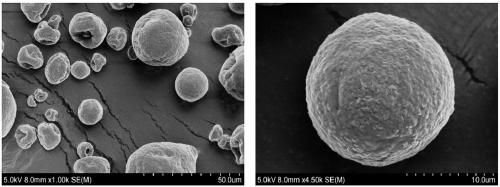
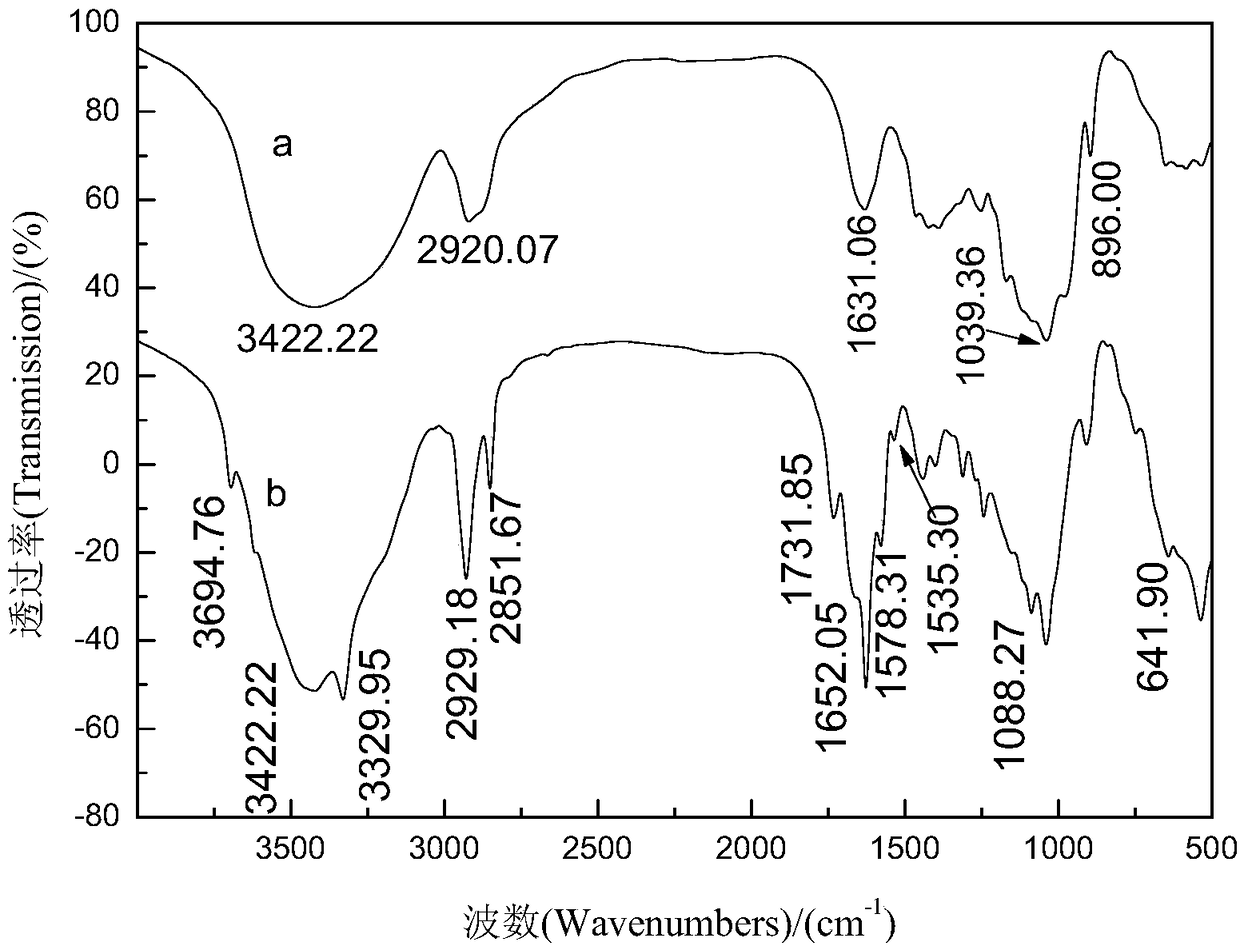
Synthesis method of bagasse xylan o-methylbenzoate-g-AM (Acrylamide)/MMA (Methyl Methacrylate)
A technology of methyl benzoate and synthesis method, which is applied in the directions of drug combination and antitumor drug, can solve the problems of complex structure, insufficient functionality, low stability of xylan, etc., and achieves improved water solubility and raw material utilization. The effect of high rate and easy control of process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0036] (1) Dry 10 g of bagasse xylan in a constant-temperature vacuum oven at 60° C. for 24 hours to constant weight to obtain dry-based bagasse xylan.
[0037] (2) Weigh 0.6g of ammonium persulfate and 0.5g of sodium bisulfite in a 50mL beaker, then add 20mL of deionized water to make an initiator solution for later use.
[0038] (3) Weigh 4gAM and 5mL of analytically pure MMA into another 250mL flask, then add 30mL of deionized water, 0.2g of N,N'-methylene bisacrylamide and 1.1g of industrial grade OP-10, and use high shear Stir and disperse in an emulsifier for 1.5 hours, prepare a mixed monomer solution, pour it into a 100mL constant pressure dropping funnel, and set aside.
[0039] (4) Weigh 5 g of dry bagasse xylan obtained in step (1) and add it into a 250 mL four-neck flask, then add 20 mL of deionized water, raise the temperature to 50° C., stir and disperse for 20 minutes. Begin to add dropwise the mixed monomer solution of step (3) gained, control dropwise in 6 ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com