Preparation method of phenolic hydroxy autopolymerization hydrogel
A technology of self-polymerization of phenolic hydroxyl groups, which is applied in the field of preparation of phenolic hydroxyl self-polymerization hydrogels, can solve problems such as complex conditions, difficulty in double bond polymerization, difficulty in double bond hydrogel polymerization, etc., and achieve good biocompatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
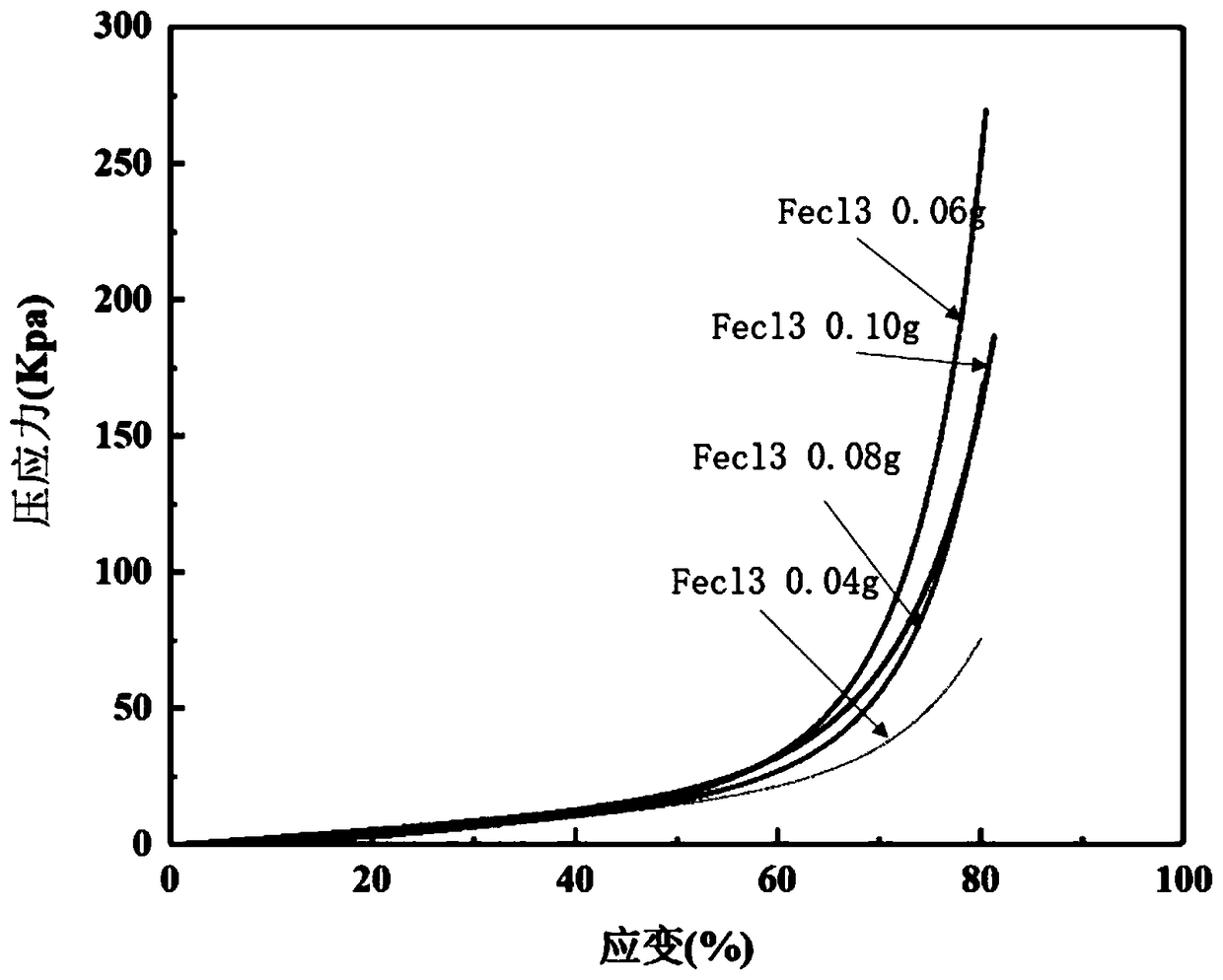
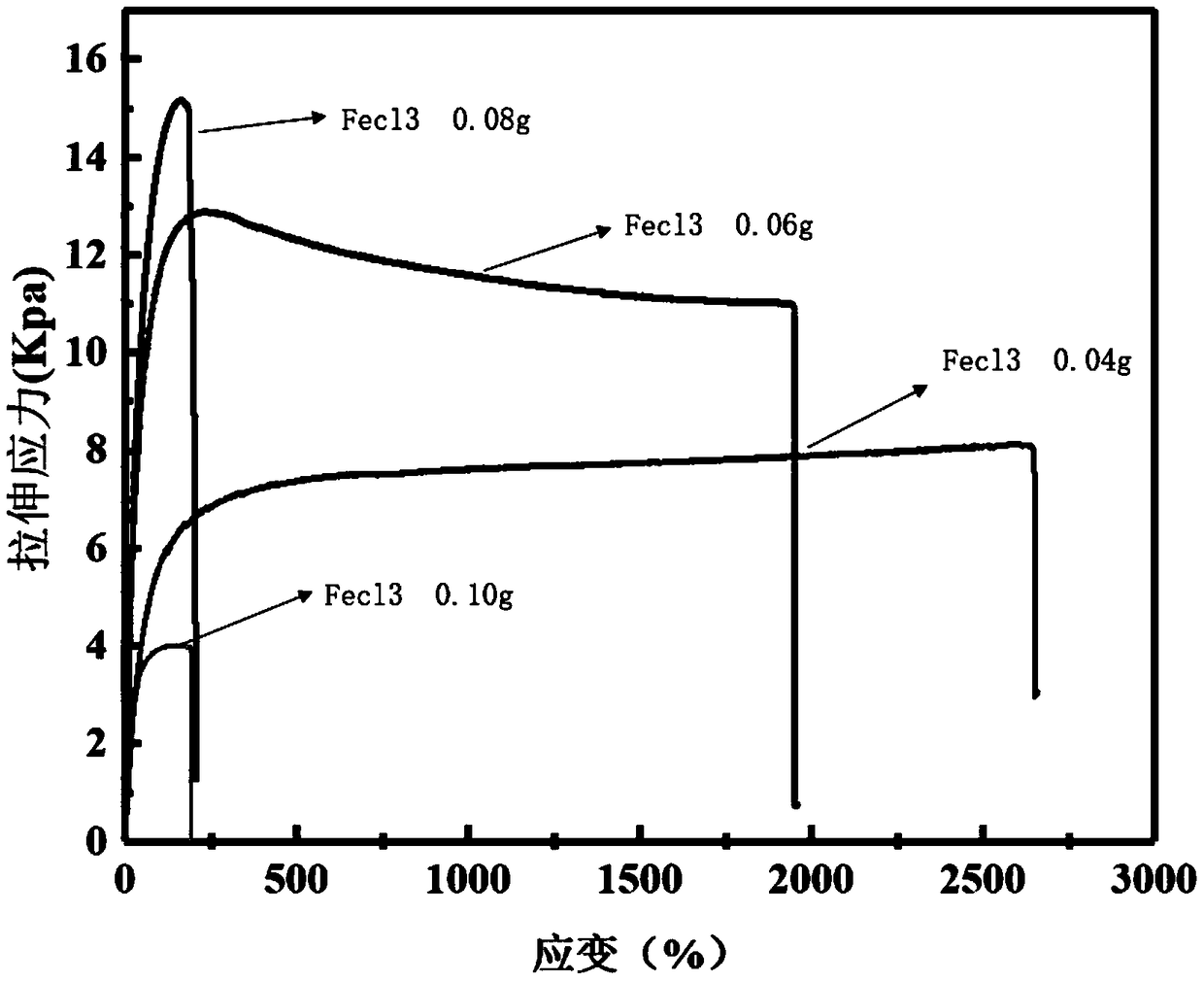
[0032] Add 0.003g of dopamine to 10ml of aqueous solution containing 0.06g of ferric chloride hexahydrate, stir evenly, and let it stand for 10s; add 2.7ml of acrylic acid, stir evenly, add 0.015g of ammonium persulfate, stir evenly, and let stand to form a gel.
[0033] Its hydrogel structure is as image 3 As shown, it can be seen from the figure that the hydrogel has a uniform porous structure inside, a smooth sheet structure, and a good distribution of internal crosslinks.
Embodiment 2
[0035] Add 0.003g of dopamine to 10ml of aqueous solution containing 0.10g of ferric chloride hexahydrate, stir evenly, and let stand for 10s; add 2.7ml of acrylic acid, stir evenly, add 0.015g of ammonium persulfate, stir evenly, and then stand still to form a gel.
Embodiment 3
[0037] Add 0.003g of dopamine to 10ml of aqueous solution containing 0.08g of ferric chloride hexahydrate, stir evenly, and let stand for 10s; add 2.7ml of acrylic acid, stir evenly, add 0.015g of ammonium persulfate, stir evenly, and let stand to form a gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com