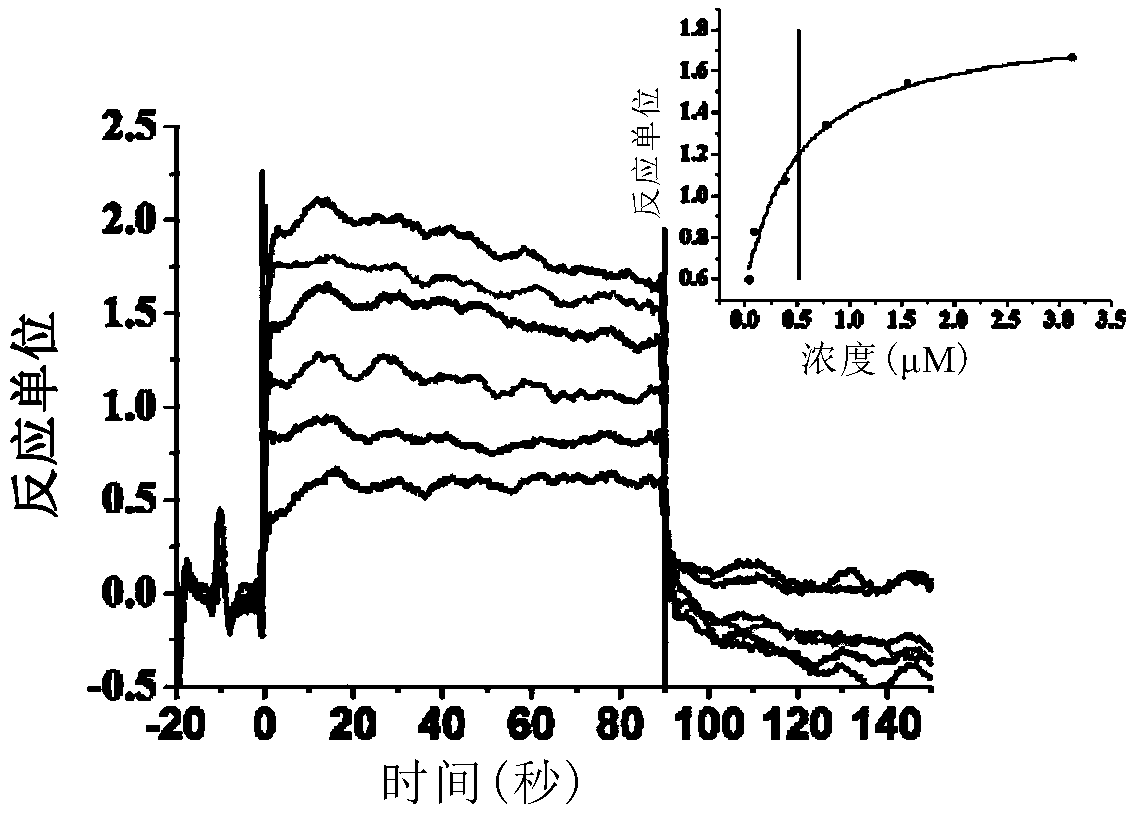
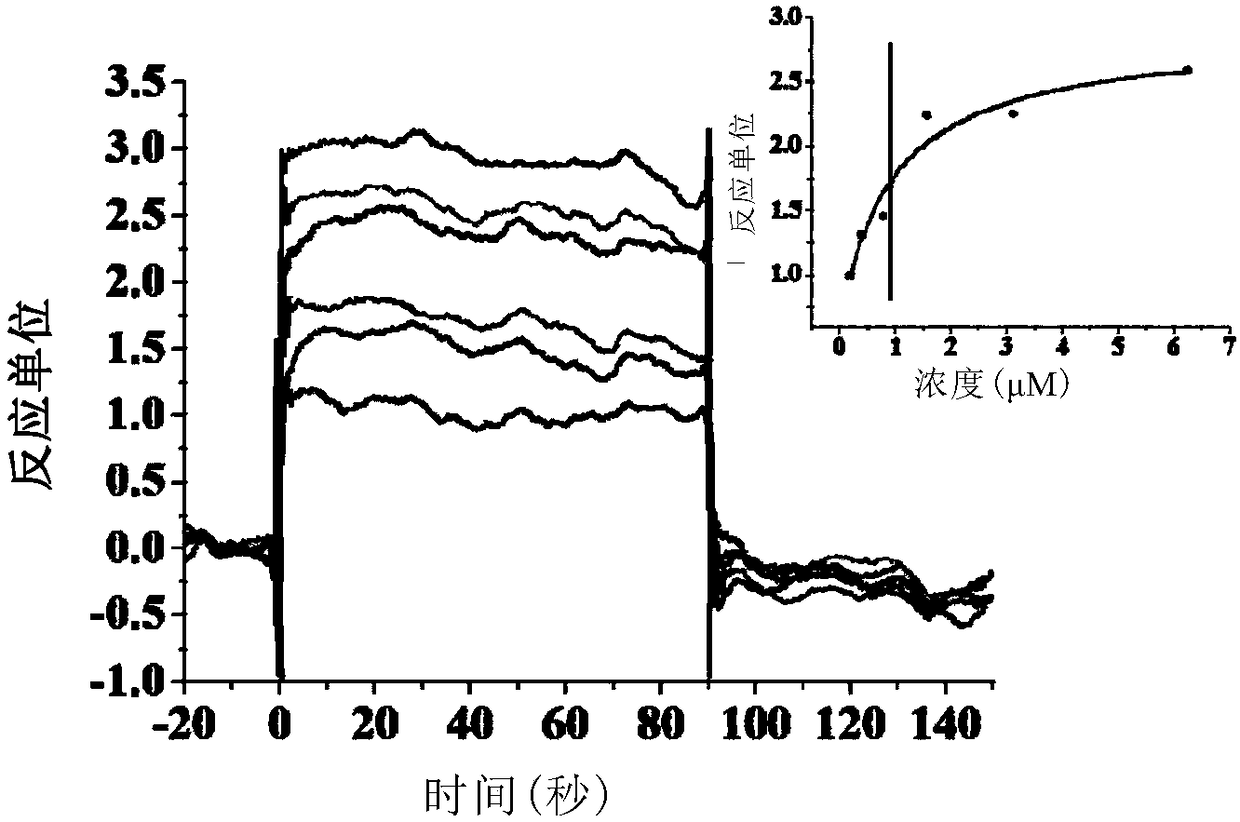
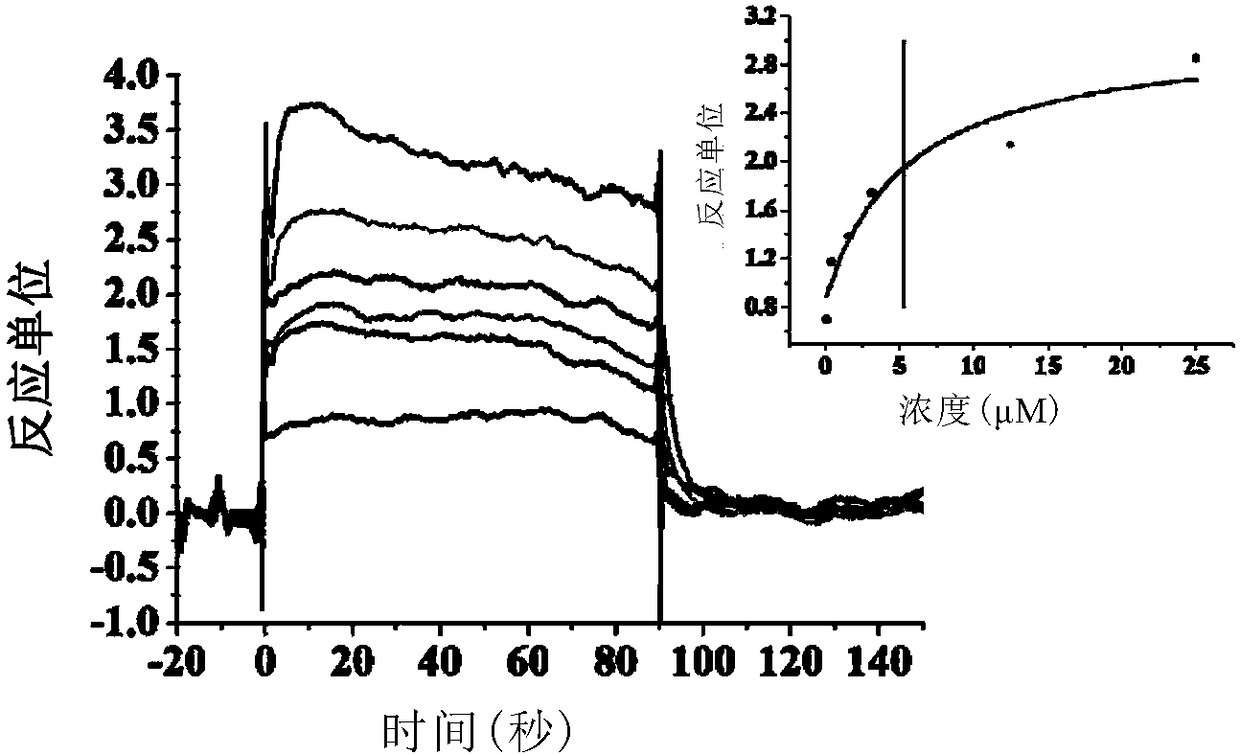
CD47/SIRP (signal regulatory protein) alpha blocking agent and application thereof
A technology for use, α protein, applied in the field of compounds that block the interaction between SIRPα protein and CD47, which can solve the problems of easy immunogenicity and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Example 1. Synthesis of 1.1-p-fluorophenyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline ethyl acetate (D3)
[0158] 1. The synthetic route of 1.1-p-fluorophenyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline ethyl acetate (D3) is as follows:
[0159]
[0160] Experimental steps:
[0161] 1) Synthesis of ethyl 2,4-dichloro-5-fluorobenzoylacetate
[0162]
[0163] First put NaH (466mg, 24.3mmol) into a 100mL dry three-neck flask, add anhydrous THF and diethyl carbonate to dissolve, protect with argon, stir at 45°C for 30 minutes, slowly add 2,4-di Chloro-5-fluoroacetophenone (1g, 4.9mmol) in diethyl carbonate solution, keep the internal temperature at 50-55°C, drop it, stir for 5 hours, the reaction is complete, pour the reaction solution into ice water containing acetic acid , extracted with ethyl acetate, and passed through a silica gel column (PE:DCM=15:1) to obtain 0.65 g of a yellow oil with a yield of 48%. The input amount obtained 4.1...
Embodiment 2
[0173] Example 2. Synthesis of 1-p-fluorophenyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline acetic acid methyl ester (D2)
[0174]
[0175] Put the above raw material drug (500mg, 1.2mmol) in a 50mL reaction bottle, add methanol 10mL and concentrated sulfuric acid 1.5mL, heat to reflux, and the reaction is complete after 5 hours. Under ice bath, add NaOH to neutralize to pH = 8-9, add DCM to extract, and spin dry the organic phase to obtain 360 mg of white solid with a yield of 76.1%. 1 H NMR (400MHz, DMSO-d 6 )δ8.39(s,1H),7.80(d,J=13.6Hz,1H),7.76(dd,J1=4.8Hz,J2=4Hz,2H),7.52(t,J=8.8Hz,2H), 6.26 (d, J=7.2Hz, 1H), 3.72 (s, 3H), 2.88-2.86 (m, 4H), 2.78-2.77 (m, 4H).
Embodiment 3
[0176] Example 3.1-Synthesis of 1-p-fluorophenyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline acetamide (D4)
[0177]
[0178] Put the methyl ester product (50mg, 0.13mmol) in a 25mL reaction flask, add n-butylamine to dissolve, stir at 66°C, and complete the reaction in 6 hours. The solvent was spin-dried to obtain 40mg of a yellow product with a yield of 72.7%. 1 H NMR (400MHz, CD 3 OD) δ8.63(s, 1H), 7.95(d, J=13.6Hz, 1H), 7.64(dd, J1=4.8Hz, J2=4.4Hz, 2H), 7.45(t, J=8.4Hz, 2H ),6.42(d,J=7.2Hz,1H),3.43(t,J=6.8Hz,2H),3.09-3.07(m,4H),3.01-2.98(m,4H),1.65-1.58(m, 2H), 1.50-1.41 (m, 2H), 0.98 (t, J=7.6Hz, 3H). HRMS(ESI)(m / z):[M+H]+calcd for C 24 h 27 f 2 N 4 o 2 , 441.2102; found, 441.2100.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com