Micro-sphere double-antibody sandwich detection method and kit for detecting soluble FAM19A4 protein
A FAM19A4, 1. FAM19A4 technology, applied to the quantitative detection method and kit for detecting soluble FAM19A4 protein, FAM19A4 protein as a disease diagnostic marker field, can solve the problem that it is difficult to meet the detection requirements of a small amount of samples, and the sample size is large
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Materials and Methods
[0070] 1. Preparation of recombinant protein
[0071] The eukaryotic recombinant proteins FAM19A1-myc-his, FAM19A4-myc-his, FAM19A5-myc-his and LYG1-myc-his were prepared according to conventional eukaryotic recombinant protein preparation methods, and the concentration was determined by the BCA quantitative method to be not less than 1mg / mL.
[0072] 2. Antibodies
[0073]Rabbit anti-human FAM19A4 polyclonal antibody was obtained by immunizing rabbits with FAM19A4-myc-his eukaryotic recombinant protein. the migration and phagocytosis of macrophages, Cell.Mol.Immunol., 2015, Volume 12, pages 615-624). Mouse anti-human FAM19A4 monoclonal antibody was used to immunize BALB / c nude mice with FAM19A4-myc-his eukaryotic recombinant protein, and 12 hybridoma clones were obtained by ELISA screening. The affinity and specificity of the 12 antibody strains were identified by Western blot and flow cytometry analysis, and the best clone was s...
Embodiment 2
[0089] Example 2 Establishment of standard curve for detection of soluble FAM19A4 protein by microsphere double-antibody sandwich method
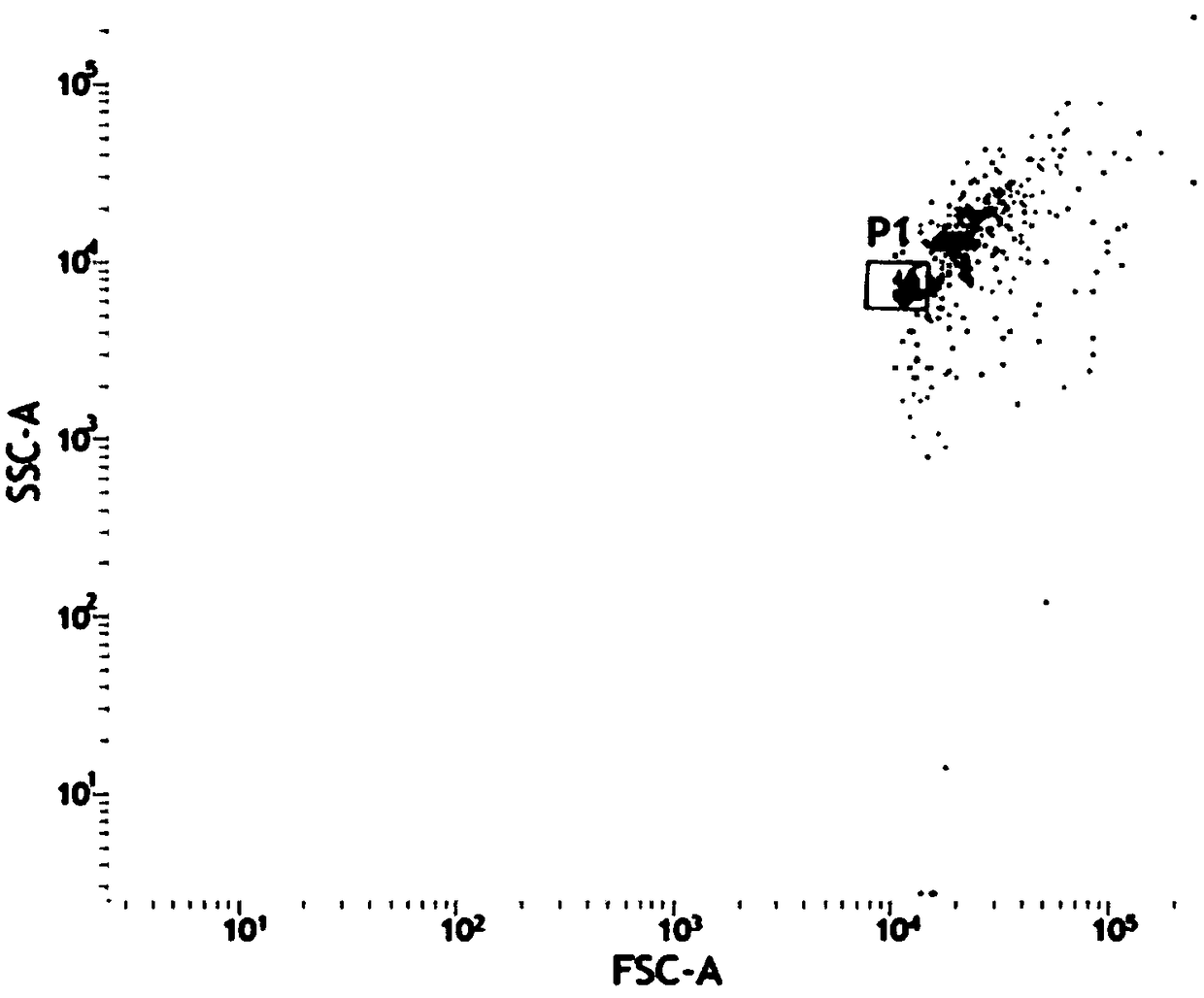
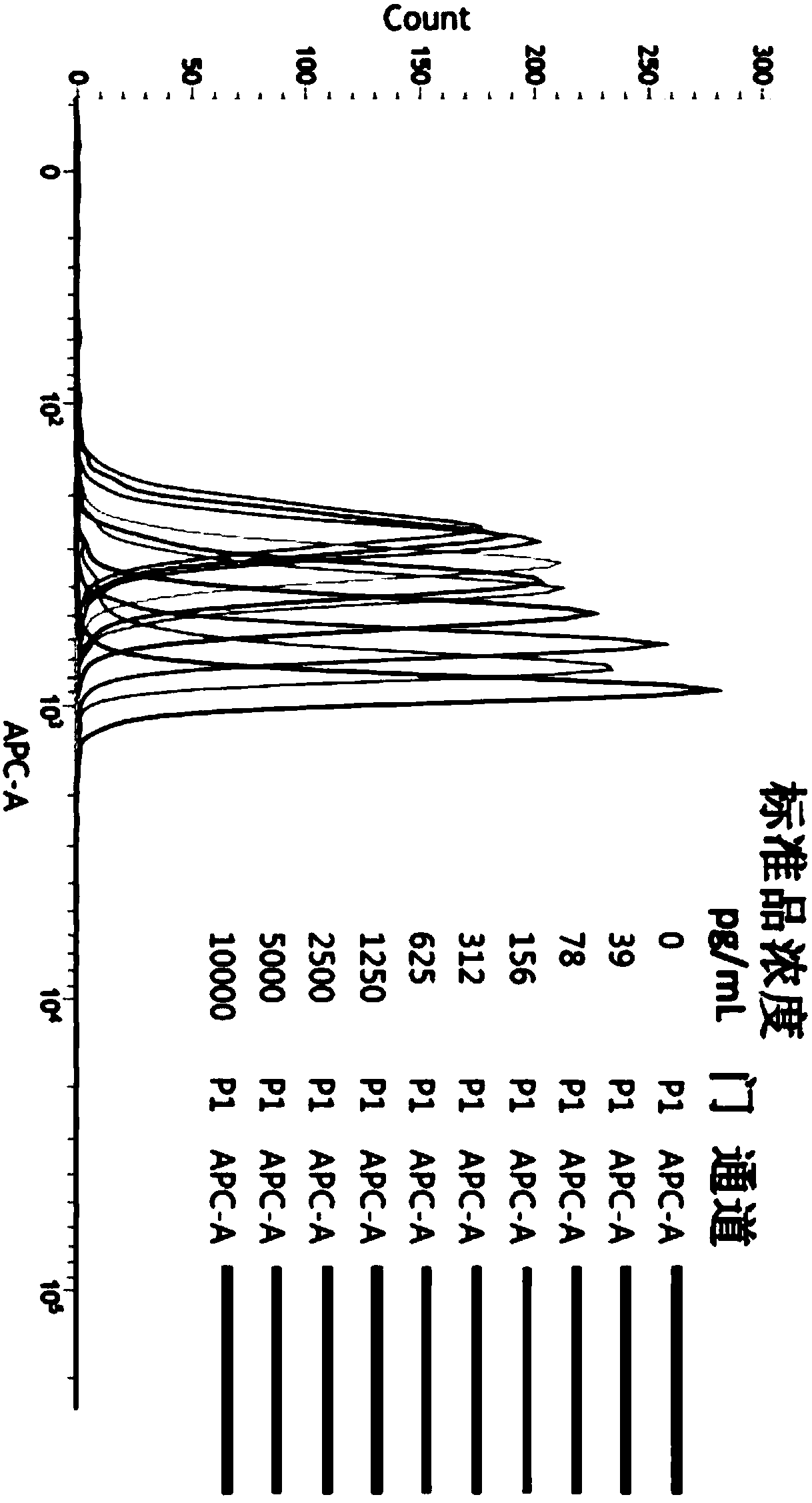
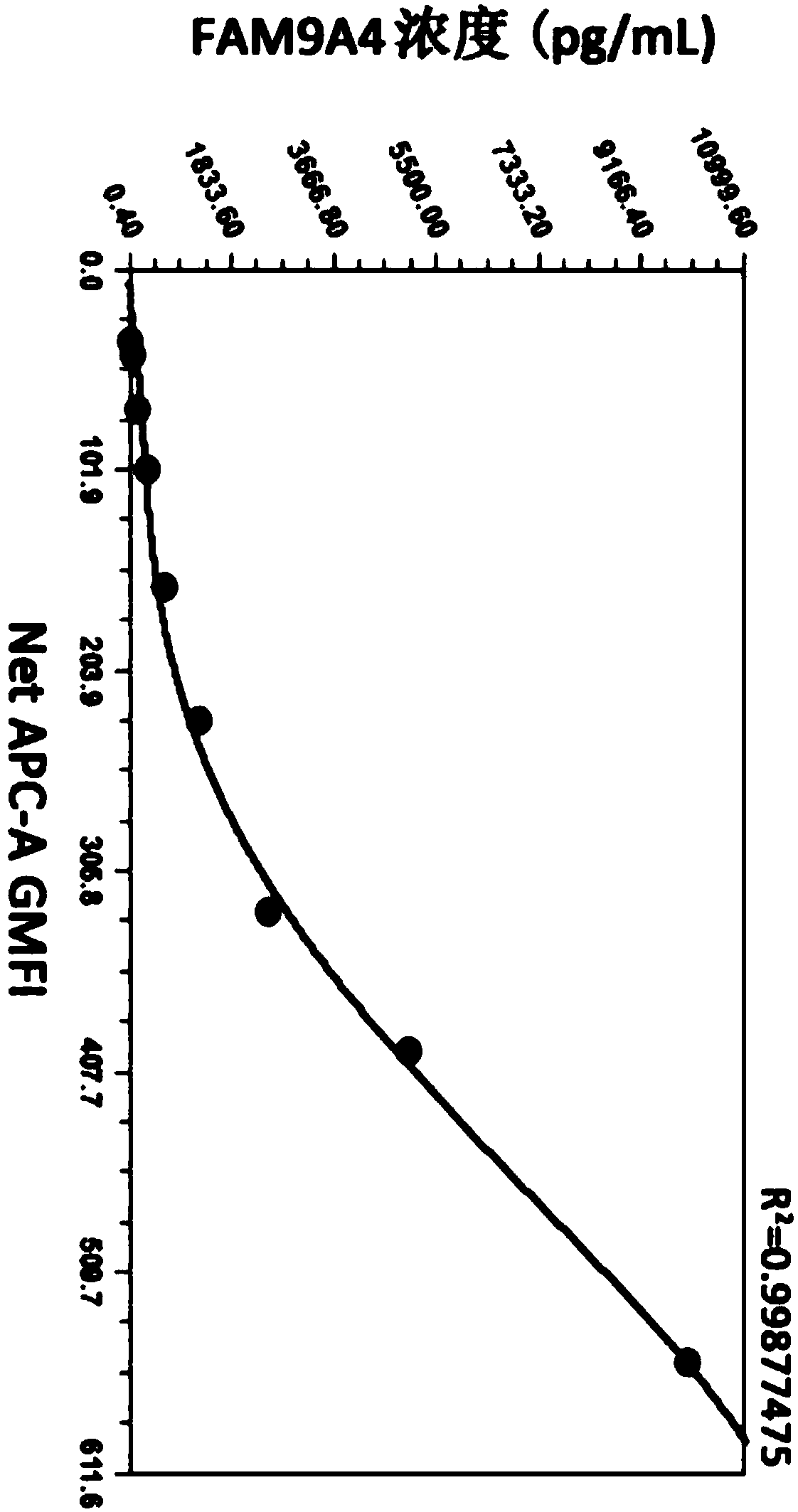
[0090] First, dilute FAM19A4-myc-his eukaryotic recombinant protein to 10ng / mL with detection dilution buffer, and then double dilution to obtain concentrations of 10ng / mL, 5ng / mL, 2.5ng / mL, 1.25ng / mL, 0.625ng / mL mL, 0.312ng / mL, 0.156ng / mL, 0.078ng / mL, and 0.039ng / mL standard gradient solutions. Test the dilution buffer as a blank control. The above-mentioned standard substance was detected according to the detection steps in Example 1, and the APC-A GMFI data was collected, and a standard curve was drawn using CurveExpert 1.4 software corresponding to the concentration of the standard substance. figure 2 Shown in Table 1 is a representative data, according to which the standard curve drawn is as image 3 shown.
[0091] Table 1
[0092]
[0093]
Embodiment 3
[0094] Example 3 Specificity, recovery and precision of detection of soluble FAM19A4 protein by microsphere double-antibody sandwich method
[0095] The method of the present invention verifies its specificity by detecting FAM19A1 and FAM19A5. Both belong to the TAFA family and share 74% and 68% sequence similarity with FAM19A4, respectively. Prepare 10 000 and 1 000 pg / mL FAM19A1 and FAM19A5 standard substances respectively, use the method of the present invention to detect, and the results are all lower than the detection limit (Table 2). This shows that there is no obvious cross-reaction between FAM19A4 and its analogues, and the method of the present invention is highly specific.
[0096] Add a certain concentration of FAM19A4 standard protein into different substrates to be tested, use the method of the present invention to detect, compare the detected concentration with the actual concentration, and calculate the recovery rate of the detection method. As shown in Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com