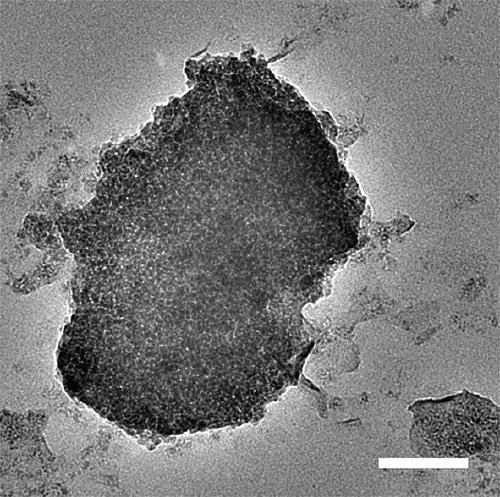
TiO2-Ti3C2-CoSx nanocrystal photocatalyst and preparation method thereof
A tio2-ti3c2-cosx, nanocrystal technology, which is applied in the field of semiconductor photocatalyst preparation, can solve the problems of limited transport, separation and utilization efficiency of photogenerated carriers, limited hydrogen evolution efficiency, etc. Efficiency, the effect of improving transmission and utilization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Amorphous TiO 2 Preparation: Add 5 mL of isopropyl titanate to 100 mL of methanol, stir at room temperature for 5 minutes, slowly add 2.5 mL of deionized water dropwise while stirring, and wait until the titanium salt is fully hydrolyzed to form a milky white suspension. Liquid centrifugation to obtain a white precipitate, and then the white precipitate was washed with ethanol for 2 to 3 times, and then washed with deionized water, and then the white precipitate was dried at 50 ° C to obtain amorphous TiO 2 .
[0025] (2) Ti 3 C 2 Preparation of: 0.5 g Ti was stripped with 5 mL of HF (49 wt%) 3 AlC 2 The Al layer in the solution was stirred at room temperature for 6 hours. After the Al layer was completely peeled off, the black suspension was centrifuged to obtain a black precipitate, and then the black precipitate was washed with deionized water for 4 to 5 times, and then the precipitate was placed in the Dry at 60°C to get Ti 3 C 2 .
[0026] (3) TiO 2 -T...
Embodiment 2
[0029] (1) Amorphous TiO 2 Preparation: Add 10mL of isopropyl titanate to 100mL of methanol, stir at room temperature for 10min, slowly add 20ml of deionized water dropwise while stirring, until the titanium salt is fully hydrolyzed, a milky white suspension is formed, and the suspension Centrifuge to obtain a white precipitate, then wash the white precipitate with ethanol for 2 to 3 times, then wash with deionized water, and then dry the white precipitate at 80°C to obtain amorphous TiO 2 .
[0030] (2) Ti 3 C 2 Preparation: 0.5 g Ti was exfoliated with 10 mL of HF (49 wt%) 3 AlC 2 The Al layer in the solution was stirred at room temperature for 24 hours. After the Al layer was completely peeled off, the black suspension was centrifuged to obtain a black precipitate, and then the black precipitate was washed with deionized water for 4 to 5 times, and then the precipitate was placed in the Dry at 80°C to get Ti 3 C 2 .
[0031] (3) TiO 2 -Ti 3 C 2 -CoS x Preparatio...
Embodiment 3
[0034] (1) Amorphous TiO 2 Preparation: Add 10mL tetrapropyl titanate to 100mL methanol, stir at room temperature for 15min, slowly add 40ml deionized water dropwise while stirring, wait until the titanium salt is fully hydrolyzed, a milky white suspension is formed, and the suspension Centrifuge to obtain a white precipitate, then wash the white precipitate with ethanol for 2 to 3 times, then wash with deionized water, and then dry the white precipitate at 100°C to obtain amorphous TiO 2 .
[0035] (2) Ti 3 C 2 Preparation of: 0.5 g Ti was stripped with 10 mL of HF (49 wt%) 3 AlC 2 The Al layer in the solution was stirred at room temperature for 35 hours. After the Al layer was completely peeled off, the black suspension was centrifuged to obtain a black precipitate, and then the black precipitate was washed with deionized water for 4 to 5 times, and then the precipitate was placed in the Dry at 100°C to get Ti 3 C 2 .
[0036] (3) TiO 2 -Ti 3 C 2 -CoS x Preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com