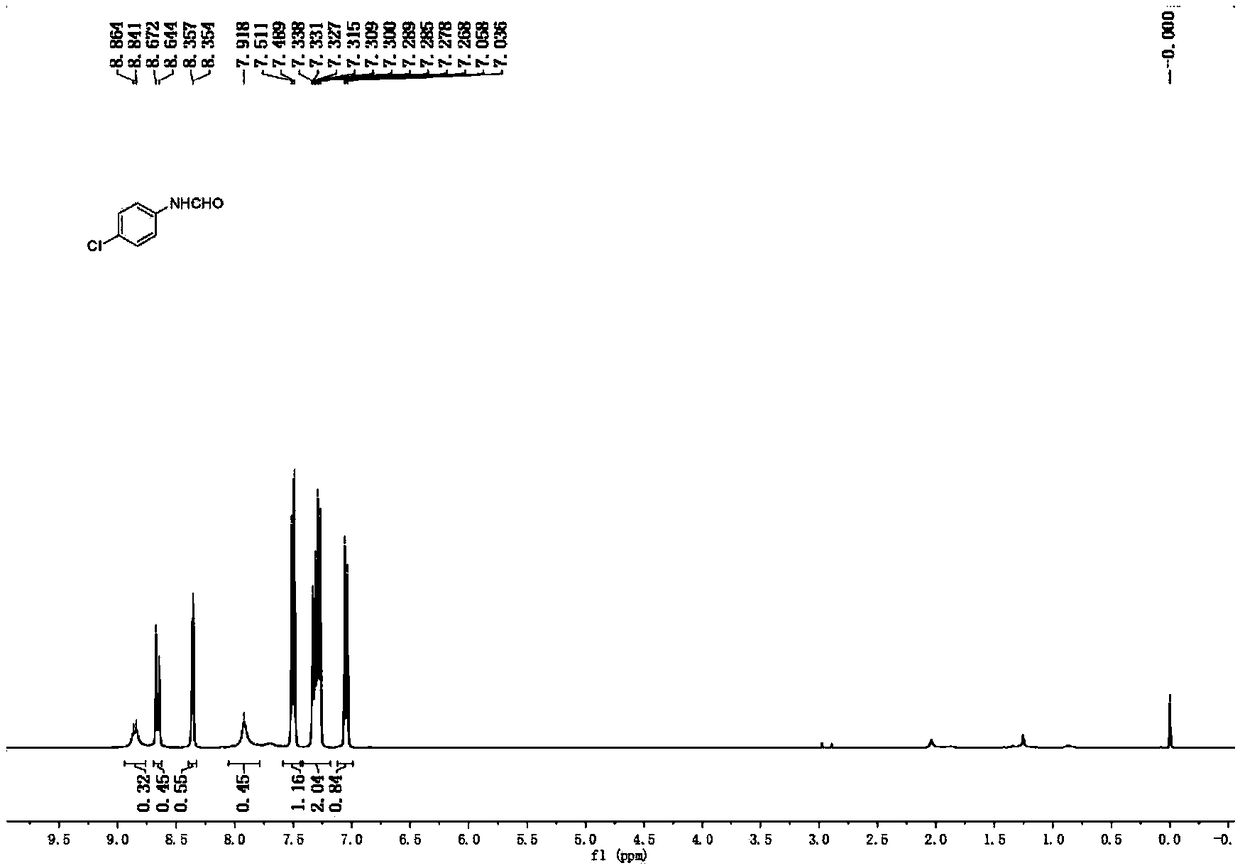
Transamination reaction of aromatic amine and amide derivatives under condition without catalysts and accelerants
A technology of amide derivatives and catalysts is applied in the field of synthesis of formamide derivatives, can solve problems such as toxicity, and achieve the effects of overcoming high toxicity, simple operation and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
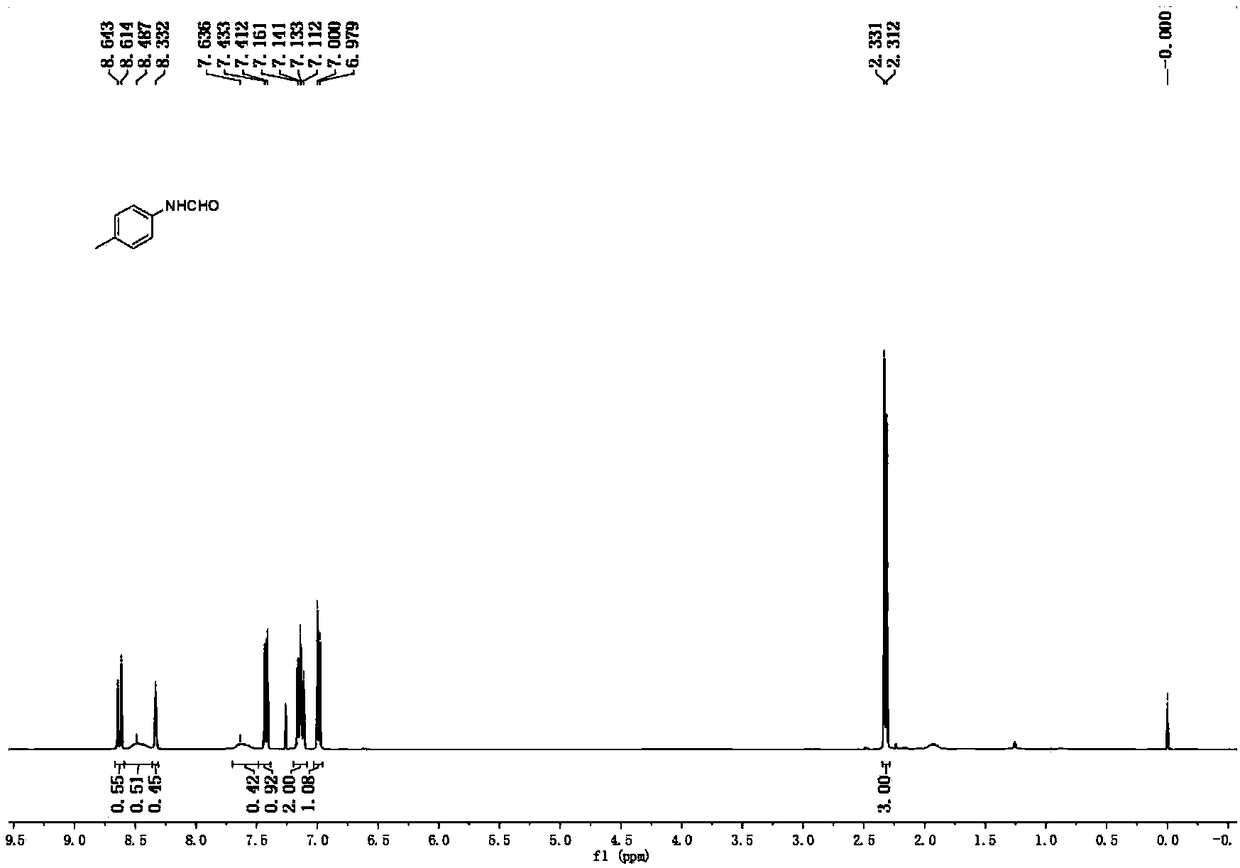
[0032] Put 4-methylaniline (107.2mg, 1.0mmol), formamide (0.4mL, 10.0mmol), and a stirring bar into the reaction tube, and seal the reaction tube under air condition. The reaction tube was placed in an oil bath reaction pot at 150°C, stirred and reacted for 24 hours, cooled to room temperature, diluted with 15 mL of water, and extracted three times with ethyl acetate, 15 mL each time. The extracts were combined, dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure, and the crude product was subjected to column chromatography with ethyl acetate: petroleum ether = 1:3 (containing 1% triethylamine) as the eluent Get pure. Yellow solid, melting point 54-56°C, yield 89%. 1 H NMR (400 MHz, CDCl 3 )δ8.63 (major rotamer, d, J=11.6Hz, 0.55H), 8.49 (major rotamer, br s, 0.51H), 8.33 (minor rotamer, s, 0.45H), 7.64 (minor rotamer, br s, 0.42H), 7.42(d, J=8.4Hz, 0.92H), 7.16-7.11 (m, 2.00H), 6.99(d, J=8.4Hz, 1.08H), 2.32(d, J=7.6Hz, 3....
Embodiment 2
[0034] Put 3-methylaniline (107.3 μL, 1.0 mmol), formamide (0.4 mL, 10.0 mmol), and a stirring bar into a reaction tube, and seal the reaction tube under air condition. The reaction tube was placed in an oil bath reaction pot at 150°C, stirred and reacted for 24 hours, cooled to room temperature, diluted with 15 mL of water, and extracted three times with ethyl acetate, 15 mL each time. The extracts were combined, dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure, and the crude product was subjected to column chromatography with ethyl acetate: petroleum ether = 1:3 (containing 1% triethylamine) as the eluent Get pure. Yellow oil, 80% yield. 1 H NMR (400MHz, CDCl 3 ) δ9.32(major rotamer,br s,0.51H),8.68(major rotamer,d,J=11.2Hz,0.54H),8.52(minor rotamer,br s,0.46H),8.30(minor rotamer,d,J =2.0Hz,0.46H),7.40(s,0.47H),7.34(d,J=8.0Hz,0.49H),7.22-7.15(m,1.00H),6.97(d,J=7.6Hz,0.53H ),6.93-6.90(m,1.51H),2.30(d,J=13.2Hz,3.00H); ...
Embodiment 3
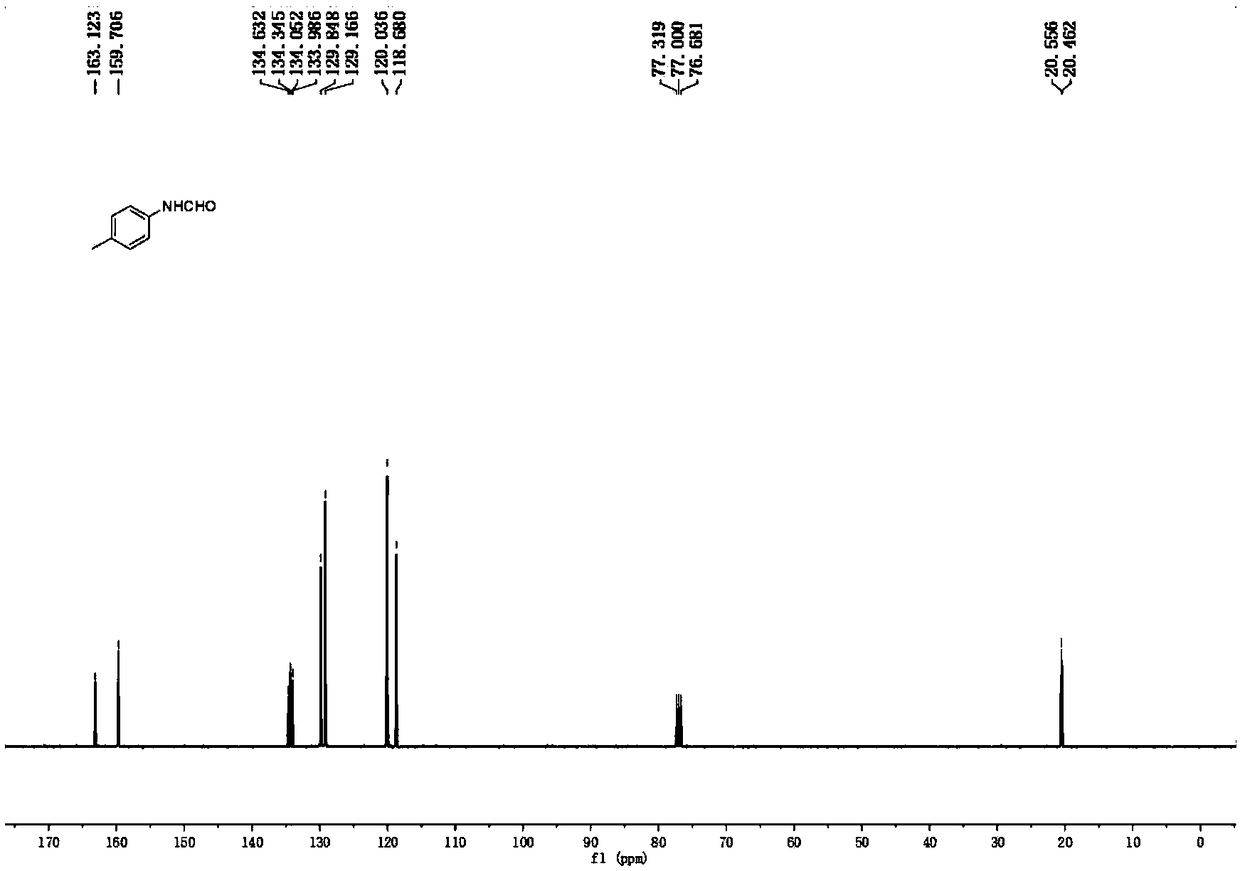
[0036] Aniline (93 μL, 1.0 mmol), formamide (0.4 mL, 10.0 mmol), and a stir bar were put into a reaction tube, and the reaction tube was sealed under air condition. The reaction tube was placed in an oil bath reaction pot at 150°C, stirred and reacted for 24 hours, cooled to room temperature, diluted with 15 mL of water, and extracted three times with ethyl acetate, 15 mL each time. The extracts were combined, dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure, and the crude product was subjected to column chromatography with ethyl acetate: petroleum ether = 1:2 (containing 1% triethylamine) as the eluent Get pure. Yellow oil, yield 83%. 1 H NMR (400MHz, CDCl3) δ8.70 (major rotamer, d, J = 11.6Hz, 0.53H), 8.39 (minor rotamer, s, 0.47H), 8.14 (major rotamer, br, 0.49H), 7.56-7.54 (m,0.90H),7.39-7.32(m,2.39H),7.20(t,J=7.4Hz,0.55H),7.15(t,J=7.6Hz,0.50H),7.11-7.09(m,1.05 H); 13 C NMR (100MHz, CDCl 3 )δminor rotamer 163.1, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com