Empagliflozin preparation method
A technology for raw materials and compounds, applied in the field of preparation of empagliflozin, can solve problems such as unfavorable environmental protection, complicated operation, etc., and achieve the effects of being beneficial to environmental protection, good in purity and yield, and reducing the generation of waste water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
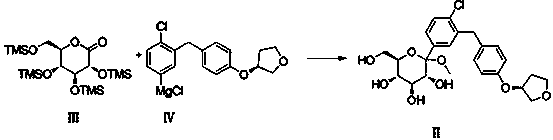
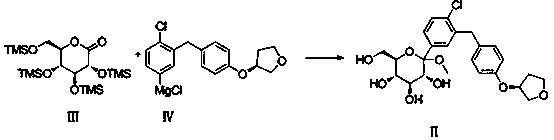
[0026] Embodiment 1: Preparation of Compound II
[0027] Compound IV obtained in Test Example 1 was lowered to -25°C, and compound III (prepared according to Test Example 2) was slowly added dropwise. After the drop was complete, the temperature was raised to -5°C, and stirring was continued at this temperature for 1 hour. After the reaction was completed, 22 Kg of methanol was added dropwise, stirred for 0.5 hours, 17 Kg of cation exchange resin (Dowex50WX8-400-H+) was added, the temperature was raised to 25°C, the reaction was stirred for 12 hours and filtered, and the filtrate was concentrated under reduced pressure to obtain light yellow viscous The thick oil was directly used in the next reaction without purification.
Embodiment 2
[0028] Embodiment 2: the preparation of compound I
[0029] Add 205 Kg of anhydrous acetonitrile, 36 Kg of anhydrous AlCl3, and 45 Kg of triethylsilane into the reaction kettle and cool down to -10°C with stirring. The oil obtained in Example 1 was dissolved in 140 Kg of dichloromethane, and was dropped into the above mixture. After the drop was completed, the temperature was raised to 20° C., and the reaction was kept for 1 hour. After the reaction was completed, the temperature was lowered to 0°C, 230 Kg of water (temperature control ≤ 10°C) was slowly added to the reaction liquid, concentrated under reduced pressure, 275 Kg of isopropyl acetate was added, and concentrated under reduced pressure. Add 275 Kg of isopropyl acetate to the above concentrate, raise the temperature to 45°C, and keep stirring for 0.5 hours. The temperature was slowly lowered to 10° C. within 3 hours, and stirring was continued for 4 hours. After filtering, the filter cake was rinsed with a small a...
Embodiment 3
[0030] Embodiment 3: Preparation of Compound II
[0031] Compound IV obtained in Test Example 1 was lowered to -20°C, and compound III (prepared according to Test Example 2) was slowly added dropwise. After the drop was complete, the temperature was raised to -5°C, and stirring was continued at this temperature for 1 hour. After the completion of the reaction, add methanol 218Kg dropwise, stir for 0.5 hour, add cation exchange resin (Dowex50WX8-400-H + ) 6.5 Kg, heated up to 60°C, and reacted for 0.5 hours. After filtration, the filtrate was concentrated under reduced pressure to obtain a light yellow viscous oil, which was directly used in the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com