A kind of injectable paclitaxel gel with high drug load and its preparation method and application
A high-drug-loading, paclitaxel-based technology, applied in pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the loss of drug activity, reduce drug loading, increase system complexity, etc. problems, achieve high drug loading, avoid high manufacturing difficulty, and retain drug activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
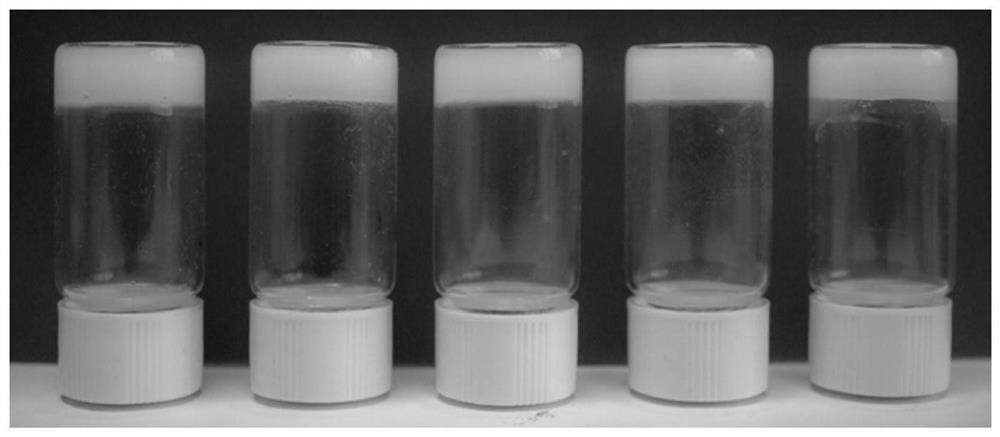
[0051] Weigh 8.8mg, 20mg, 40mg, 80mg, and 135mg of paclitaxel respectively, and dissolve them in turn in screw bottles containing 1mL of PEG400. After ultrasonically dissolving at room temperature, slowly add 0.75mL, 0.70mL, and 0.55mL to each screw bottle, respectively. , 0.33mL, 0.10mL of PBS phosphate-buffered saline, after ultrasonic dispersion again, let stand at room temperature for a period of time, the paclitaxel content is respectively 0.522% (5mg / mL), 1.22% (10mg / mL), 2.44% (25mg / mL), 5.63% (60mg / mL), and 11.33% (120mg / mL) paclitaxel gels, the gel formation photos of the inverted observation method are as follows figure 1 shown.
Embodiment 2
[0053] Weigh 50 mg of paclitaxel and add it to a screw bottle of 1 mL of ethanol. After ultrasonic dispersion at room temperature, add 0.1 mL of PBS phosphate-buffered saline solution dropwise. (45mg / mL) gel, paclitaxel gel SEM photo as figure 2 shown.
Embodiment 3
[0055] Weigh 60 mg of paclitaxel and add it to a threaded bottle containing 1 mL PEG200. After ultrasonic dispersion at room temperature, slowly add 0.3 mL of 5% glucose injection. 3.99% (42 mg / mL) gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com