Synthesis method and application of avermectin B2-based emamectin benzoate
A technology of emamectin and avermectin, applied in the field of organic synthesis, can solve the problems of great difference in activity and failure to be effectively utilized, and achieve low raw material cost, significant market advantage, and insecticidal activity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The present embodiment based on Abamectin B 2 The preparation method of emamectin benzoate, described method comprises the steps:
[0050] (1) Hydroxyl protection reaction
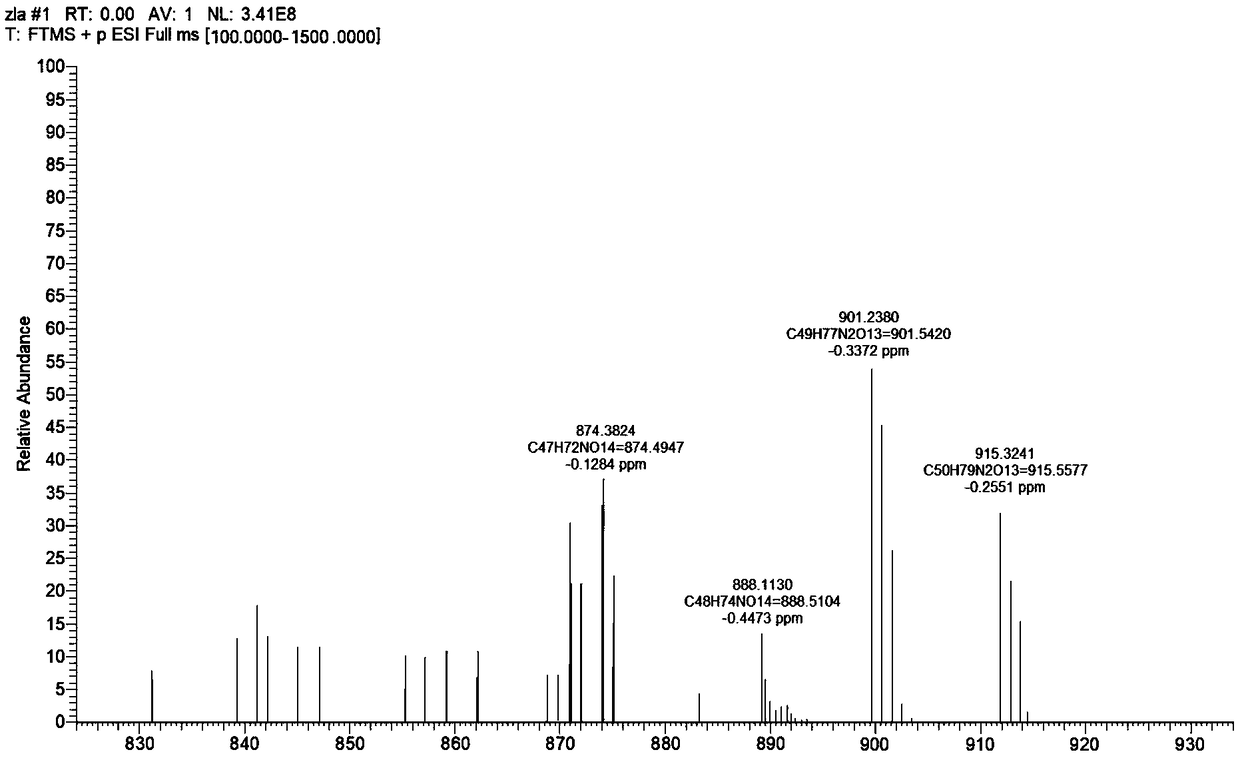
[0051] Abamectin B 2 As a raw material, the hydroxyl groups at the 5th and 23rd positions were selectively protected by allyl chloroformate to obtain the intermediate I (5-allyl carboxyl abamectin B 2 and 5,23-dicarboxylate abamectin B 2 ).
[0052] Concrete reaction steps are briefly described as: 1mol avermectin B 2 Dissolve in dichloromethane, under the protection of argon, add 12 mol of triethylamine, then dropwise add 2 mol of allyl chloroformate, continue to pass in argon for 1 hour after the dropwise addition, and react in a closed system for 48 hours. After the reaction was completed, the solvent was removed to obtain the crude product, which was washed with dichloromethane and acetone to obtain the intermediate I (5-allyl carboxyl abamectin B 2 and 5,23-dicarboxylate abamectin B 2 )....
Embodiment 2
[0068] The present embodiment based on Abamectin B 2 The preparation method of emamectin benzoate, described method comprises the steps:
[0069] (1) Hydroxyl protection reaction
[0070] Abamectin B 2 As a raw material, the 5-position and 23-position hydroxyl groups are selectively protected by allyl chloroformate to obtain intermediate I (5-allyl carboxyl abamectin B 2 and 5,23-dicarboxylate abamectin B 2 ).
[0071] Concrete reaction steps are briefly described as: 10mmol abamectin B 2 Dissolve in dichloromethane, under the protection of argon, add 120mmol of triethylamine, then dropwise add 20mmol of allyl chloroformate, after the dropwise addition, continue to flow argon for 1 hour, then close the system for reaction for 48 hours. After the reaction was completed, the solvent was removed to obtain the crude product, which was washed with dichloromethane and acetone to obtain the intermediate I (5-allyl carboxyl abamectin B 2 and 5,23-dicarboxylate abamectin B 2 ). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com