The preparation method of domperidone tablet
A technology for dipperidone tablets and domperidone, applied to non-active ingredient medical preparations, active ingredient-containing medical preparations, pharmaceutical formulas, etc., can solve problems such as not considering the friability of plain tablets, and achieve high production efficiency , fast dissolution rate, high tablet hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
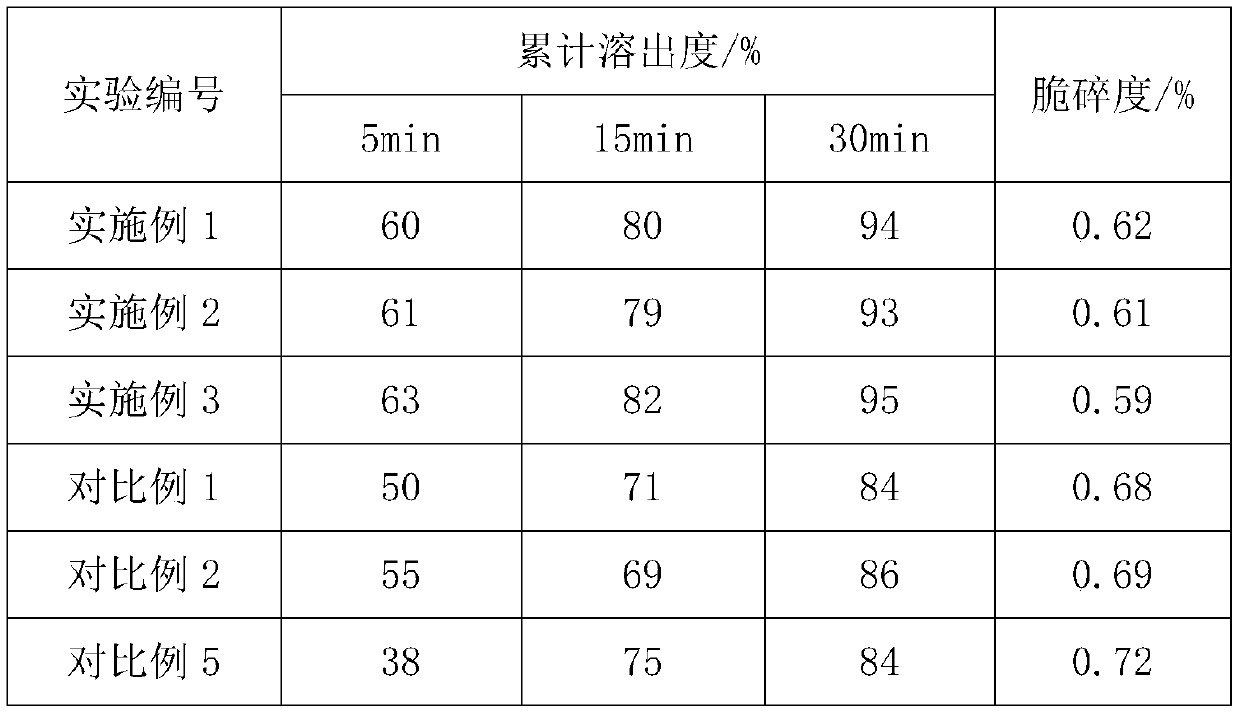
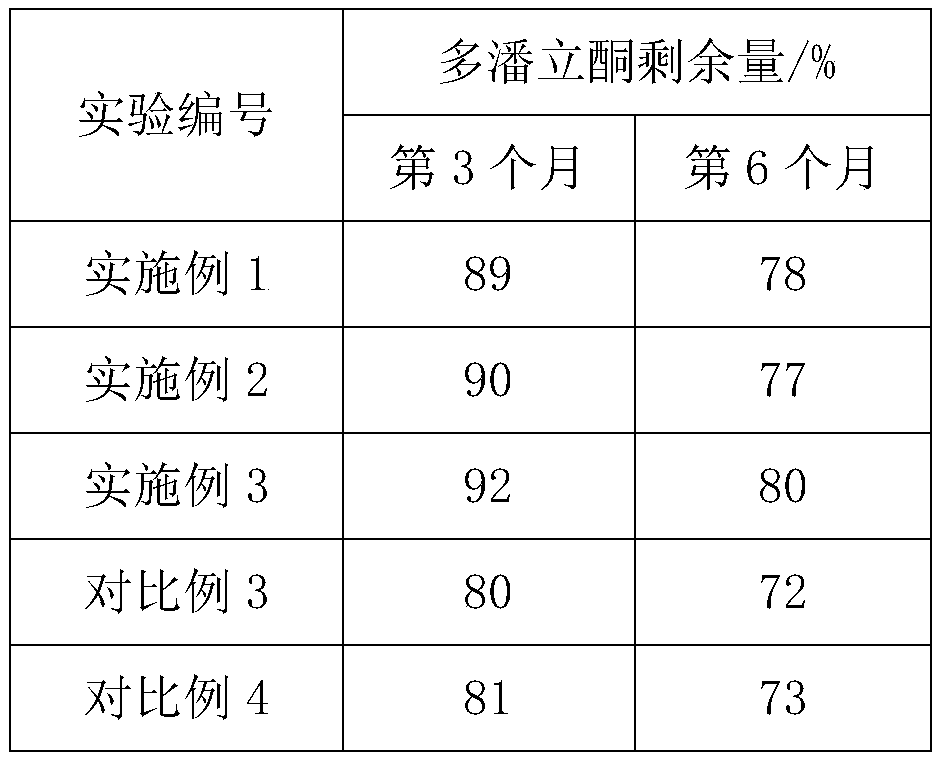
Embodiment 1
[0031] The preparation method of embodiment 1 domperidone sheet
[0032] (1) Ingredients: Clear the site for inspection and receive raw and auxiliary materials. Among them, the raw material is 4 parts of domperidone, and the auxiliary materials include 40 parts of lactose G200, 8 parts of pregelatinized starch, 5 parts of low-substituted hydroxypropyl cellulose, 2 parts of povidone K30, 0.3 parts of sodium lauryl sulfate, 0.1 parts of silicon dioxide, 1.2 parts of magnesium stearate;
[0033] (2) Granulation: with purified water, povidone K30 is mixed with the povidone K30 solution that the mass percent concentration is 20%, and the raw and auxiliary materials except magnesium stearate, povidone K30 and silicon dioxide are passed through Sieve and stir to obtain mixture 1, then slowly add povidone K30 solution and 8% purified water by mass of mixture 1, and stir evenly, sieve to make wet granules, dry, sieve and collect to obtain dry granules ;
[0034] (3) Blending: adding ...
Embodiment 2
[0036] The preparation method of embodiment 2 domperidone tablets
[0037] (1) Ingredients: Clear the site for inspection and receive raw and auxiliary materials. Among them, the raw materials are 8 parts of domperidone, and the auxiliary materials include 30 parts of lactose G200, 16 parts of pregelatinized starch, 1 part of low-substituted hydroxypropyl cellulose, 5 parts of povidone K30, 0.05 parts of sodium lauryl sulfate, 1.5 parts of silicon dioxide, 0.1 parts of magnesium stearate;
[0038] (2) Granulation: with purified water, povidone K30 is mixed with the povidone K30 solution that the mass percent concentration is 45%, and the raw and auxiliary materials except magnesium stearate, silicon dioxide and povidone K30 are passed through Sieve and stir to obtain mixture 1, then slowly add povidone K30 solution and purified water, the quality of purified water is 11% of the mass of mixture 1, stir evenly, sieve to make wet granules, dry, sieve and collect , to obtain dry ...
Embodiment 3
[0041] The preparation method of embodiment 3 domperidone tablets
[0042] (1) Ingredients: Clear the site for inspection and receive raw and auxiliary materials. Among them, the raw material is 6 parts of domperidone, and the auxiliary materials include 36 parts of lactose G200, 12 parts of pregelatinized starch, 3 parts of low-substituted hydroxypropyl cellulose, 3.6 parts of povidone K30, 0.12 parts of sodium lauryl sulfate, 0.6 parts of silicon dioxide, 0.48 parts of magnesium stearate;
[0043] (2) Granulation: with purified water, povidone K30 is mixed with the povidone K30 solution that mass percent concentration is 35%, will pass the raw and auxiliary materials except magnesium stearate, silicon dioxide and povidone K30 Sieve and stir to obtain mixture 1, then slowly add povidone K30 solution and purified water, the quality of purified water is 10% of the mass of mixture 1, stir evenly, sieve to make wet granules, dry, sieve and collect , to obtain dry particles;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com