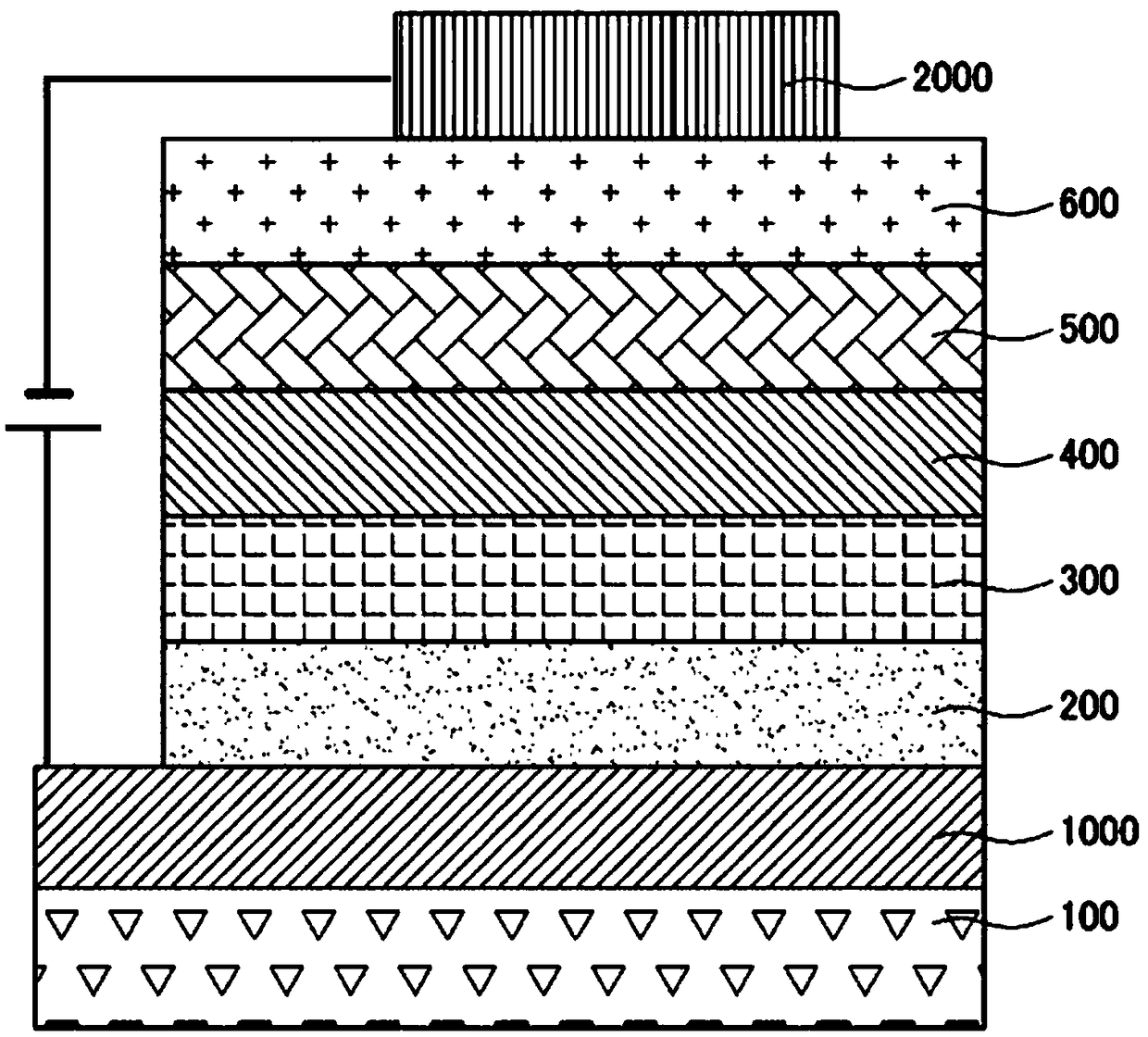
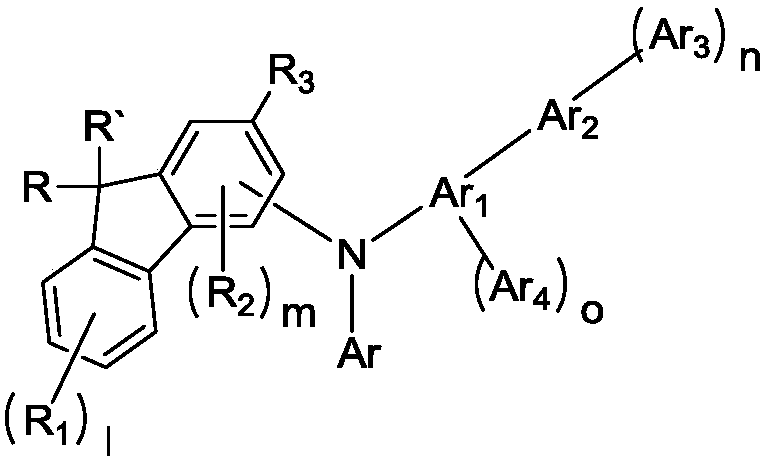
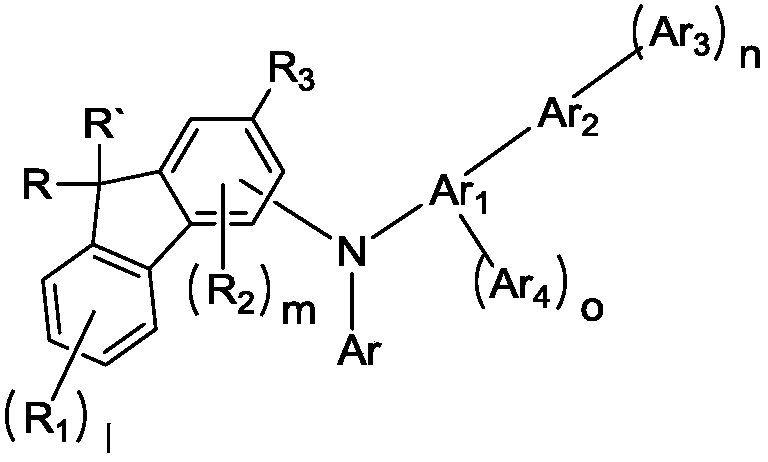
Novel compound and organic electroluminescent device including the same
A technology of organic light-emitting devices and compounds, applied in the field of novel arylamine compounds and organic light-emitting devices, can solve the problems of short life, high driving voltage, low efficiency, etc., and achieve recrystallization prevention, high color purity, and high luminous efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0150] The synthesis of preparation example 1.IM
[0151] The intermediate IM for the synthesis of the target compound can be synthesized as follows, but not limited thereto.
[0152] h = halogen atom
[0153]
[0154] h = halogen atom
[0155]
[0156] h = halogen atom
[0157] or
[0158] h = halogen atom
[0159]
[0160] The reactant IM1 used to synthesize the target compound was synthesized as follows:
[0161] The synthesis method of the following IM1 is as follows.
[0162]
[0163] In a round bottom flask, dissolve 27.6 g of [1,1'-biphenyl]-4-ylboronic acid and 50.0 g of 4-bromo-4'-iodo-1,1'-biphenyl in 1,4- Dioxane (1,4-dioxan) 800ml, put K 2 CO 3 (2M)210ml and Pd(PPh 3 ) 4 After 4.8 g, reflux and stir. The reaction was confirmed by thin layer chromatography (TLC), and the reaction was terminated after adding water. The organic layer was extracted with methylcellulose (MC), filtered under reduced pressure, and then recrystallized to obtain 15...
preparation example 2
[0167] Preparation Example 2: Synthesis of OP
[0168] The following synthesis method of OP1 is as follows.
[0169]
[0170] In a round bottom flask, 10.0 g of the above-mentioned 3-bromo-9,9-diphenyl-9H-fluorene, 3.8 g of aniline, 5.3 g of t-BuONa, Pd 2 (dba) 3 1.4g, (t-Bu) 3 1.6 ml of P was dissolved in 150 ml of toluene, and stirred under reflux. The reaction was confirmed by thin layer chromatography (TLC), and the reaction was terminated after adding water. The organic layer was extracted with methylcellulose (MC), filtered under reduced pressure, and then recrystallized to obtain 7.3 g of OP1 (yield 70%).
[0171] Using the same method as OP1 above, using different starting materials, the following OP2 to OP5 were synthesized.
[0172]
[0173]
Synthetic example 1
[0174] Synthesis Example 1. Synthesis of Compound 1
[0175]
[0176] In a round bottom flask, IM1 7.0g, OP1 5.2g, t-BuONa 2.6g, Pd 2 (dba) 3 0.7g, (t-Bu) 3 P0.7ml was dissolved in 200ml of toluene, and stirred under reflux. The reaction was confirmed by thin layer chromatography (TLC), and the reaction was terminated after adding water. The organic layer was extracted with methylcellulose (MC) and filtered under reduced pressure, followed by column purification and recrystallization to obtain 7.07 g of Compound 1 (yield 66%).
[0177] m / z: 589.28 (100.0%), 590.28 (49.1%), 591.28 (11.8%), 592.29 (1.8%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com