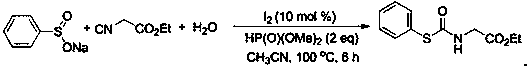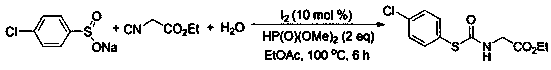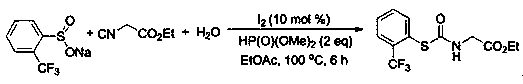A kind of preparation method of thiocarbamate compound
A thiocarbamate and compound technology, applied in organic chemistry methods, chemical instruments and methods, organic chemistry and other directions, can solve problems such as unfavorable actual production, complicated and tedious operations, unstable and easy to oxidize, etc., to avoid metal reagents. Efficacy of use, process safety, avoidance of toxic phosgene and carbon monoxide use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] At room temperature, add sodium benzene sulfinate (0.2 mmol), ethyl isonitrile (0.4 mmol), water (0.6 mmol), acetonitrile (1.5 mL), molecular iodine (0.02 mmol), And dimethyl phosphite (0.4mmol), mix well, and then at 100 o After heating and stirring the reaction for 6 hours under C condition, the reaction was detected by TLC until the completion of the reaction, 2 mL of water was added, and then extracted with ethyl acetate (4 mL) for 3 times. The extract was concentrated under reduced pressure under 0.08 Mpa to solvent-free to obtain the crude product. Then it was washed with a mixed eluent of petroleum ether and ethyl acetate in a volume ratio of 5:1, and flash column chromatography on a silica gel column to obtain 83% thiocarbamate and 39.7 mg of white solid.
[0030] 1 H NMR (500 MHz, CDCl 3 ): δ 7.61 – 7.59 (m, 2H), 7.47 – 7.42 (m, 3H),6.00 (s, 1H), 4.21 (q, J = 7.2 Hz, 2H), 4.04 (d, J = 5.1 Hz, 2H), 1.28 (t, J = 7.2 Hz, 3H); 13 C NMR (125 MHz, CDCl 3 ): ...
Embodiment 2
[0032]
[0033] At room temperature, add sodium benzene sulfinate (0.2 mmol), ethyl isonitrile (0.4 mmol), water (0.6 mmol), ethyl acetate (1.5 mL), and molecular iodine (0.02 mmol) into a 25 mL reaction tube. ), and diethyl phosphite (0.4mmol), mix well, and then at 100 o After the reaction was heated and stirred for 6 hours under C condition, the reaction was detected by TLC, 2 mL of water was added, and then extracted with ethyl acetate (4 mL) three times. The extract was concentrated under vacuum at 0.08Mpa to solvent-free to obtain the crude product. Then it was washed with a mixed eluent of petroleum ether and ethyl acetate at a volume ratio of 5:1, and flash column chromatography on a silica gel column to obtain 75% thiocarbamate and 35.9 mg of white solid.
Embodiment 3
[0035]
[0036] At room temperature, add sodium benzene sulfinate (0.2 mmol), ethyl isonitrile (0.4 mmol), water (0.6 mmol), ethyl acetate (1.5 mL), and molecular iodine (0.02 mmol) into a 25 mL reaction tube. ), and diisopropyl phosphite (0.4mmol), mix well, and then at 100 o After the reaction was heated and stirred for 6 hours under C condition, the reaction was detected by TLC, 2 mL of water was added, and then extracted with ethyl acetate (4 mL) three times. The extract was concentrated under vacuum at 0.08Mpa to solvent-free to obtain the crude product. Then it was washed with a mixed eluent of petroleum ether and ethyl acetate in a volume ratio of 5:1, and flash column chromatography on a silica gel column to obtain 60% thiocarbamate and 28.7 mg of white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com