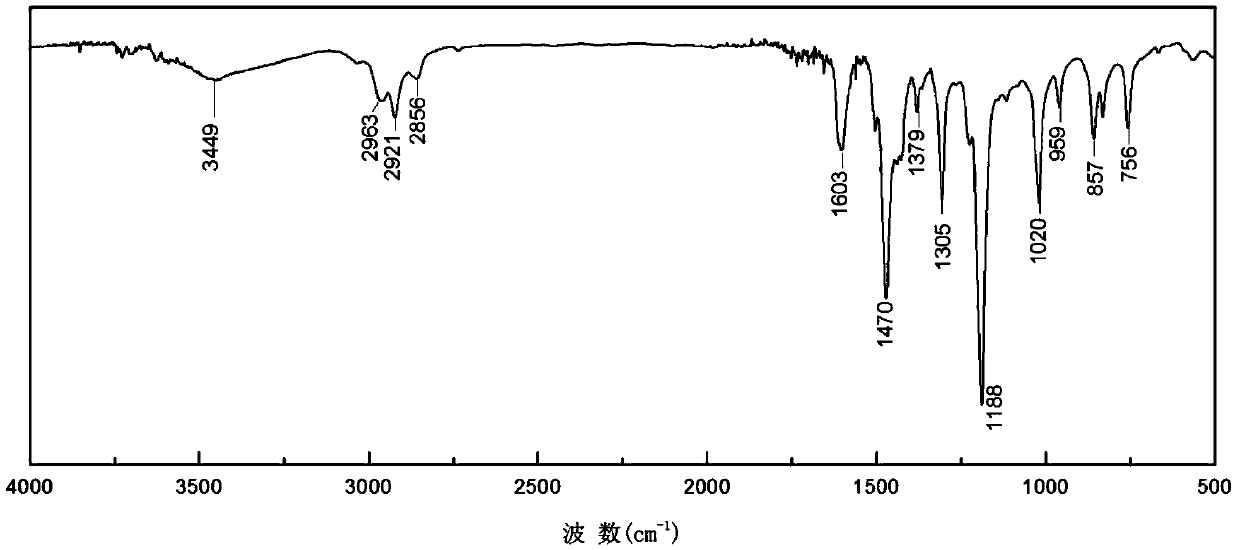
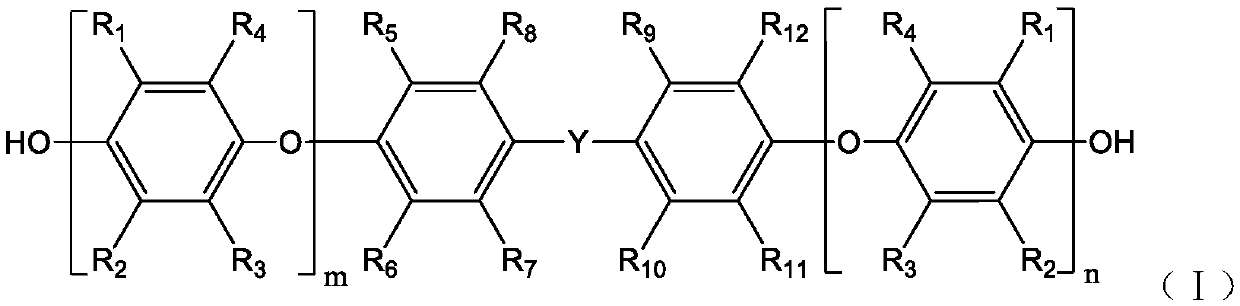
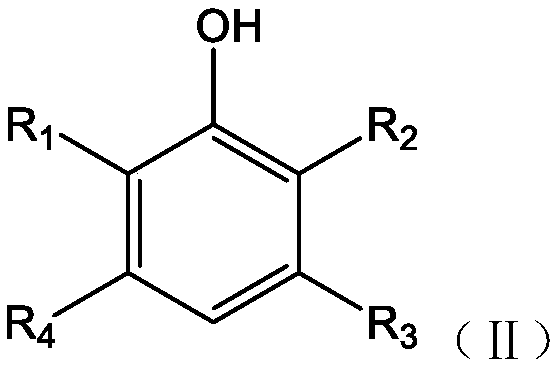
Synthesis method of double-end hydroxy polyphenylene ether oligomer
A double-ended hydroxyl polyphenylene ether, synthesis method technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve the problem of low reactivity, residual polyphenol monopoly In order to improve product quality, improve product hydroxyl content, and promote the effect of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A kind of synthetic method of double-terminated hydroxyl polyphenylene ether oligomer, comprises the following steps:
[0053] 1) Add 335.50g of 2,6-dimethylphenol, 57.00g of bisphenol A, 779.13g of toluene and 114.00g of methanol into a reaction kettle with a condensation reflux device, stir until the monomers are completely dissolved, then add 16.50g of ten Dialkylpolyethyleneimine ligands (M n =2000) and 6.17g cuprous bromide hydrobromic acid solution (freshly prepared with 0.45g cuprous oxide and 5.72g 48% hydrobromic acid in advance), mix well, feed oxygen (flow rate is 200sccm), 40 ℃ of reaction 150min;
[0054] 2) Add the reacted polymer solution into 50.81mL of 10% trisodium nitrilotriacetate solution, react at 70°C for 60min, and remove the aqueous phase by liquid-liquid centrifugation;
[0055] 3) Concentrate the oil phase obtained by centrifugation in step 2) to about 900mL, then add 9000mL of methanol to precipitate, filter, wash with methanol for 3 times,...
Embodiment 2
[0057] A kind of synthetic method of double-terminated hydroxyl polyphenylene ether oligomer, comprises the following steps:
[0058] 1) Add 305.00g of 2,6-dimethylphenol, 47.33g of tetramethylbisphenol A, 942.62g of toluene and 94.00g of methanol into a reaction kettle with a condensation reflux device, stir until the monomers are completely dissolved, then add 12.53g branched polyethyleneimine ligand (M n =600) and 4.11g cuprous bromide hydrobromic acid solution (freshly prepared with 0.30g cuprous oxide and 3.81g 48% hydrobromic acid in advance), mix well, pass into oxygen (flow rate is 200sccm), 40 ℃ of reaction 150min;
[0059] 2) Add the reacted polymer solution into 33.87mL 10% trisodium nitrilotriacetate solution, react at 70°C for 60min, and remove the water phase by liquid-liquid centrifugation;
[0060] 3) Concentrate the oil phase obtained by centrifugation in step 2) to about 900mL, then add 9000mL of methanol to precipitate, filter, wash with methanol for 3 tim...
Embodiment 3
[0062] A kind of synthetic method of double-terminated hydroxyl polyphenylene ether oligomer, comprises the following steps:
[0063] 1) Add 341.60g of 2,6-dimethylphenol, 80.00g of bisphenol F, 801.26g of toluene and 160.00g of methanol into a reaction kettle with a condensing reflux device, stir until the monomers are completely dissolved, then add 16.85g of linear Polyethyleneimine ligand (M n =1200) and 5.22g cuprous chloride hydrochloric acid solution (freshly prepared with 0.60g cuprous oxide and 4.62g 37% hydrochloric acid in advance), mix uniformly, pass into oxygen (flow rate is 200sccm), 45 ℃ of reaction 150min;
[0064] 2) Add the reacted polymer solution into 33.87mL 20% trisodium nitrilotriacetate solution, react at 70°C for 60min, and remove the aqueous phase by liquid-liquid centrifugation;
[0065] 3) Concentrate the oil phase obtained by centrifugation in step 2) to about 900mL, then add 9000mL of methanol to precipitate, filter, wash with methanol for 3 time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com