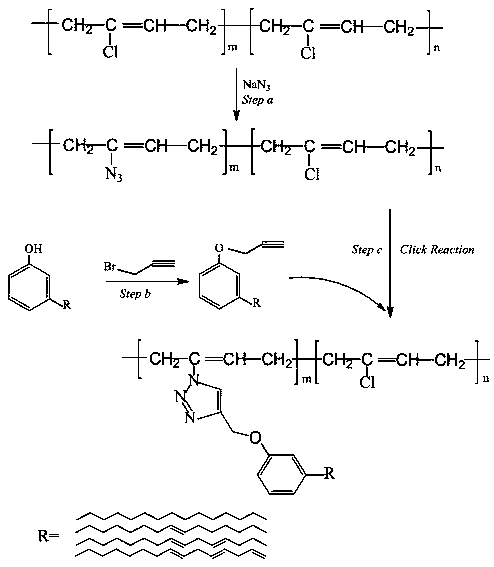
Method for turning biomass cardanol into chloroprene rubber internal plasticizer by click chemistry
A technology of neoprene and click chemistry, which is applied in the field of preparing plasticizers, can solve problems such as not causing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] (1) Synthesis of propargyl ether cardanol (CP)
[0025] Measure 5L of ethanol with a graduated cylinder and add it to a three-necked flask, then add cardanol (600g) and heat to 70°C and stir until completely dissolved. Weigh 3-bromopropyne (288g) according to the molar ratio of 3-bromopropyne and cardanol 1.2:1, weigh potassium hydroxide (134g) and dissolve it in ethanol to prepare a solution, and slowly add hydrogen into the four-neck flask Potassium oxide and 3-bromopropyne reacted at a certain temperature for 24 hours, then stopped the reaction and cooled to room temperature, and the product was distilled under reduced pressure to remove water and solvent. After vacuum drying at 55°C for 24 h, a reddish-brown viscous product was obtained.
[0026] (2) Synthesis of azide functional neoprene (CR-N 3 )
[0027] Weigh 100g of neoprene and put it into a 5L three-necked bottle, add 3L of mixed solvent and stir to dissolve. After obtaining a colloidal solution, take 50g ...
example 2
[0031] (1) Synthesis of propargyl ether cardanol (CP)
[0032] Measure 5L of ethanol with a graduated cylinder and add it to a three-necked flask, then add cardanol (470g) and heat to 70°C and stir until completely dissolved. Weigh 3-bromopropyne (148g) according to the molar ratio of 3-bromopropyne and cardanol 1.2:1, weigh potassium hydroxide (107g) and dissolve it in ethanol to prepare a solution, and slowly add hydrogen into the four-neck flask Potassium oxide and 3-bromopropyne reacted at a certain temperature for 24 hours, then stopped the reaction and cooled to room temperature, and the product was distilled under reduced pressure to remove water and solvent. After vacuum drying at 55°C for 24 h, a reddish-brown viscous product was obtained.
[0033] (2) Synthesis of azide functional neoprene (CR-N 3 )
[0034] Weigh 87g of neoprene and put it into a 5L three-neck bottle, add 3L of mixed solvent and stir to dissolve. After obtaining a colloidal solution, take 42gNaN ...
example 3
[0038] (1) Synthesis of propargyl ether cardanol (CP)
[0039] Measure 5L of ethanol with a graduated cylinder and add it to a three-necked flask, then add cardanol (752g) and heat to 70°C and stir until completely dissolved. Weigh 3-bromopropyne (302g) according to the molar ratio of 3-bromopropyne and cardanol 1.2:1, weigh potassium hydroxide (152g) and dissolve it in ethanol to prepare a solution, and slowly add hydrogen into the four-neck flask Potassium oxide and 3-bromopropyne were reacted at a certain temperature for 24 hours, then the reaction was stopped and cooled to room temperature, and the product was distilled under reduced pressure to remove water and solvent. After vacuum drying at 55°C for 24 h, a reddish-brown viscous product was obtained.
[0040] (2) Synthesis of azide functional neoprene (CR-N 3 )
[0041] Weigh 117g of neoprene and put it into a 5L three-necked bottle, add 3L of mixed solvent and stir to dissolve. After obtaining a colloidal solution, ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com