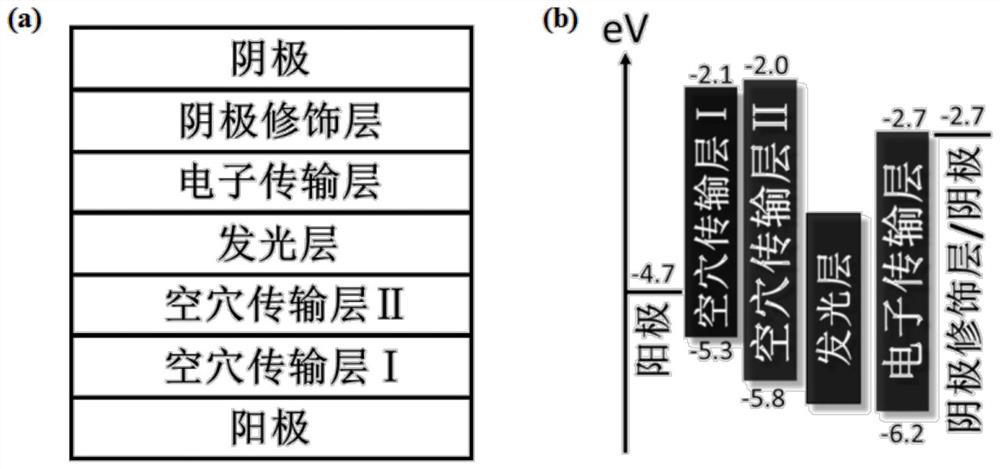
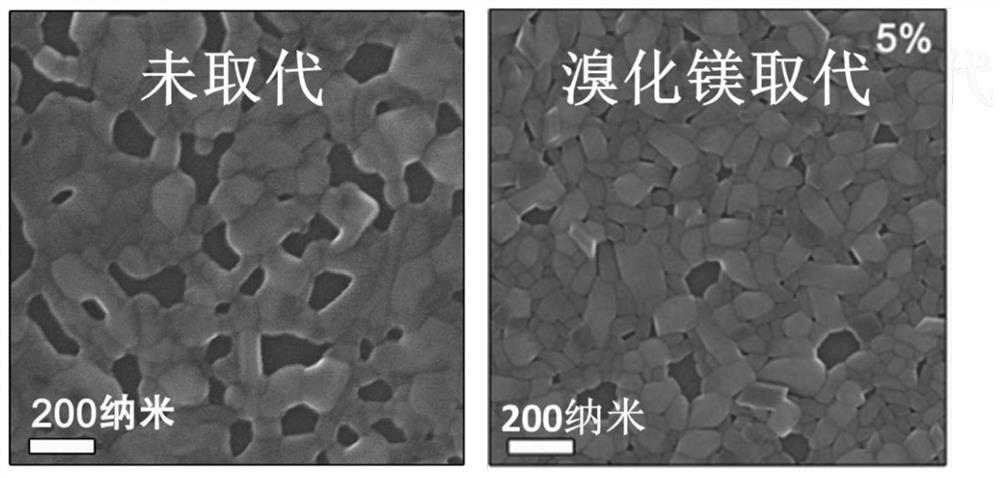
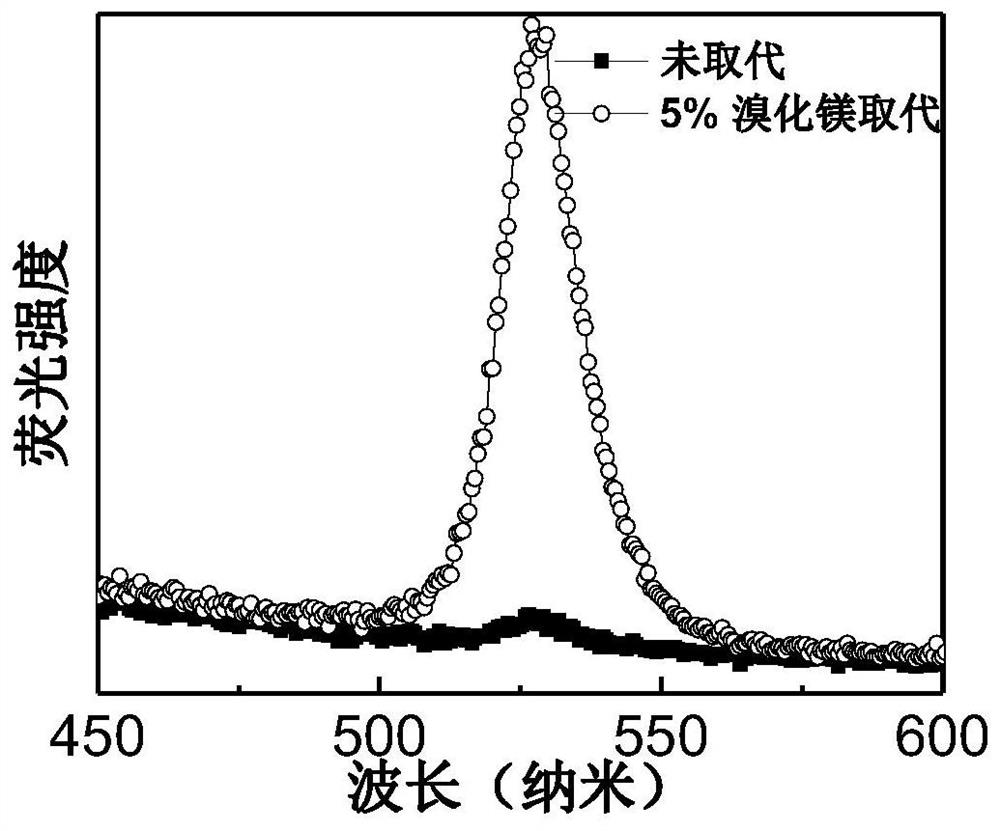
An all-inorganic lead halide perovskite light-emitting diode and its preparation method and active light-emitting layer
A light-emitting diode and lead halide technology, which is applied in the fields of organic semiconductor devices, semiconductor/solid-state device manufacturing, electric solid-state devices, etc., can solve the problem of poor stability of perovskite light-emitting diodes and easy oxidation into tetravalent tin ions and divalent tin Ion instability and other issues, to achieve the effect of film morphology improvement, fluorescence intensity improvement, not easy to oxidative denaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0054] Preparation of active light-emitting layer precursors containing alkaline earth metal bromides: MgBr 2 , CsBr and PbBr 2 Dissolved in DMSO, CsBr, MgBr in the active light-emitting layer precursor 2 and PbBr 2 The molar ratio is 1:0.05:0.95, and the concentration is 0.2M.
[0055] TFB was dissolved in chlorobenzene to obtain a first hole transport layer solution with a concentration of 8 mg / ml.
[0056] PVK was dissolved in toluene to obtain a second hole transport layer solution with a concentration of 4 mg / ml.
[0057] The solution prepared above was heated and stirred at 50° C. for 2 hours, and then used.
[0058] The ITO transparent substrate was ultrasonically cleaned with acetone, ethanol, and deionized water for 20 minutes, dried with nitrogen, and then treated with oxygen plasma for 15 minutes. The substrate was placed in a nitrogen glove box to prepare the device.
[0059] Preparation of the hole transport layer: first, the first hole transport layer soluti...
Embodiment example 2
[0066] MgBr 2 , CsBr and PbBr 2 Dissolved in DMSO to prepare an active light-emitting layer precursor containing alkaline earth metal bromide, the active light-emitting layer precursor contains CsBr, MgBr 2 and PbBr 2 The molar ratio is 1:0.1:0.9, and the concentration is 0.2M.
[0067] TFB was dissolved in chlorobenzene to obtain a first hole transport layer solution with a concentration of 8 mg / ml.
[0068] PVK was dissolved in toluene to obtain a second hole transport layer solution with a concentration of 4 mg / ml.
[0069] The solution prepared above was heated and stirred at 50° C. for 2 hours, and then used.
[0070] The ITO transparent substrate was ultrasonically cleaned with acetone, ethanol, and deionized water for 20 minutes, dried with nitrogen, and then treated with oxygen plasma for 15 minutes. The substrate was placed in a nitrogen glove box to prepare the device.
[0071] Preparation of the hole transport layer: first, the first hole transport layer soluti...
Embodiment example 3
[0078] MgBr 2 , CsBr and PbBr 2 Dissolved in DMSO to prepare an active light-emitting layer precursor containing alkaline earth metal bromide, the active light-emitting layer precursor contains CsBr, MgBr 2 and PbBr 2 The molar ratio is 1:0.2:0.8, and the concentration of the precursor solution is 0.2M.
[0079] TFB was dissolved in chlorobenzene to obtain a first hole transport layer solution with a concentration of 8 mg / ml.
[0080] PVK was dissolved in toluene to obtain a second hole transport layer solution with a concentration of 4 mg / ml.
[0081] The solution prepared above was heated and stirred at 50° C. for 2 hours, and then used.
[0082] The ITO transparent substrate was ultrasonically cleaned with acetone, ethanol, and deionized water for 20 minutes, dried with nitrogen, and then treated with oxygen plasma for 15 minutes. The substrate was placed in a nitrogen glove box to prepare the device.
[0083]Preparation of the hole transport layer: first, the first ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com