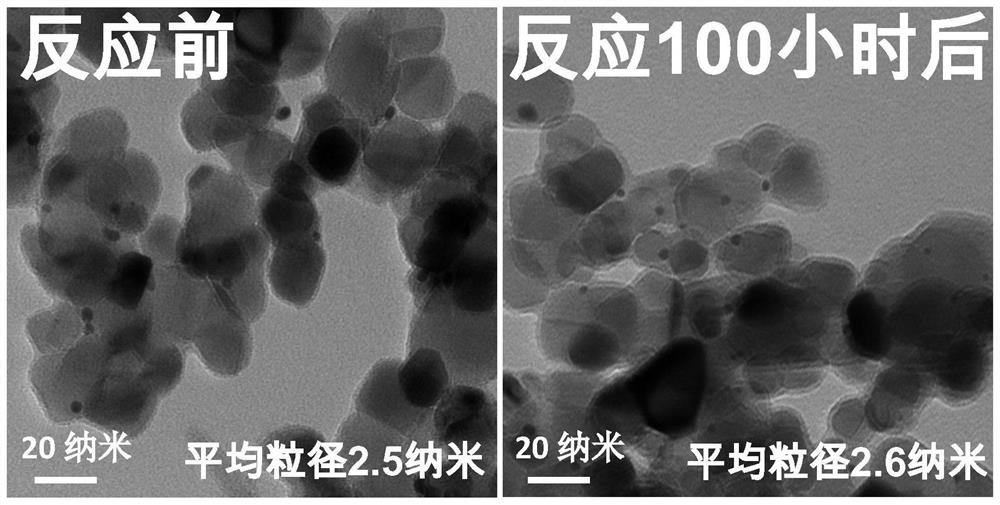
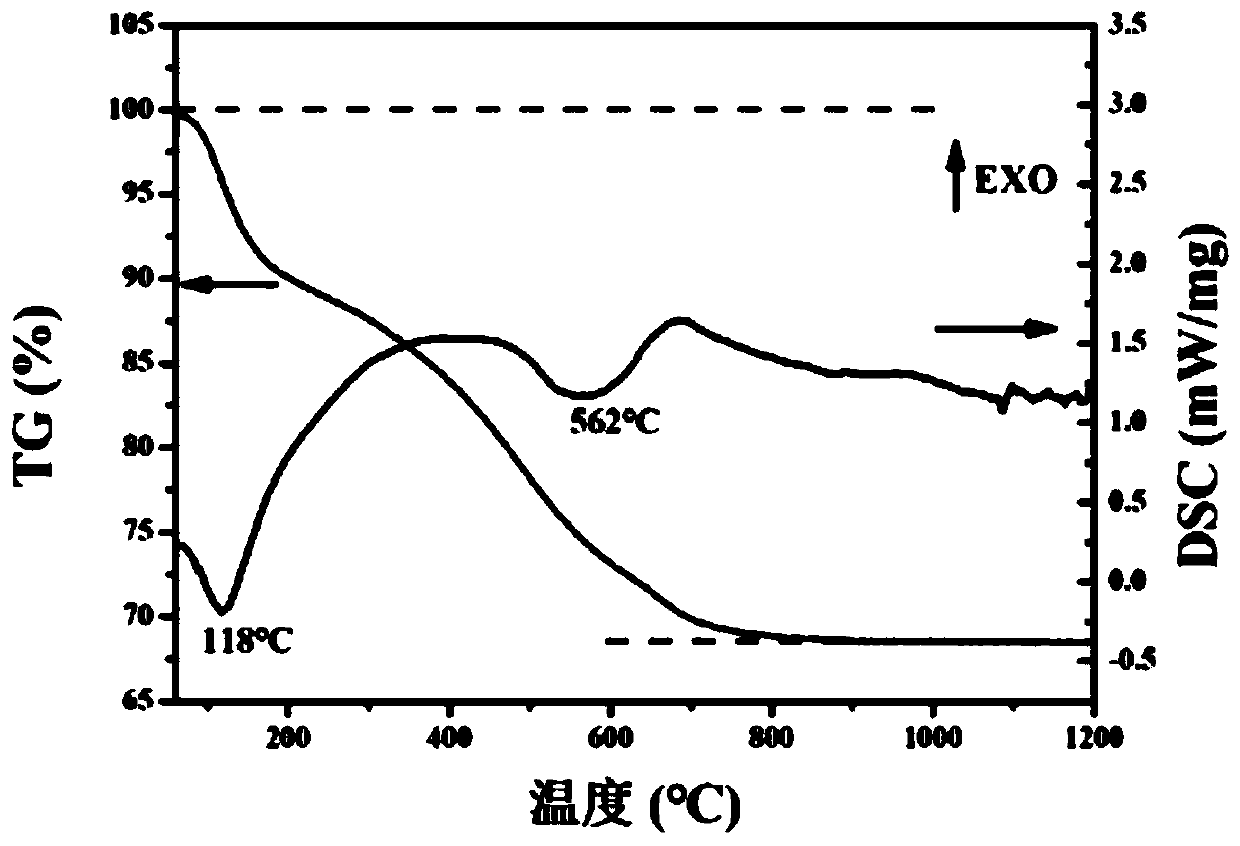
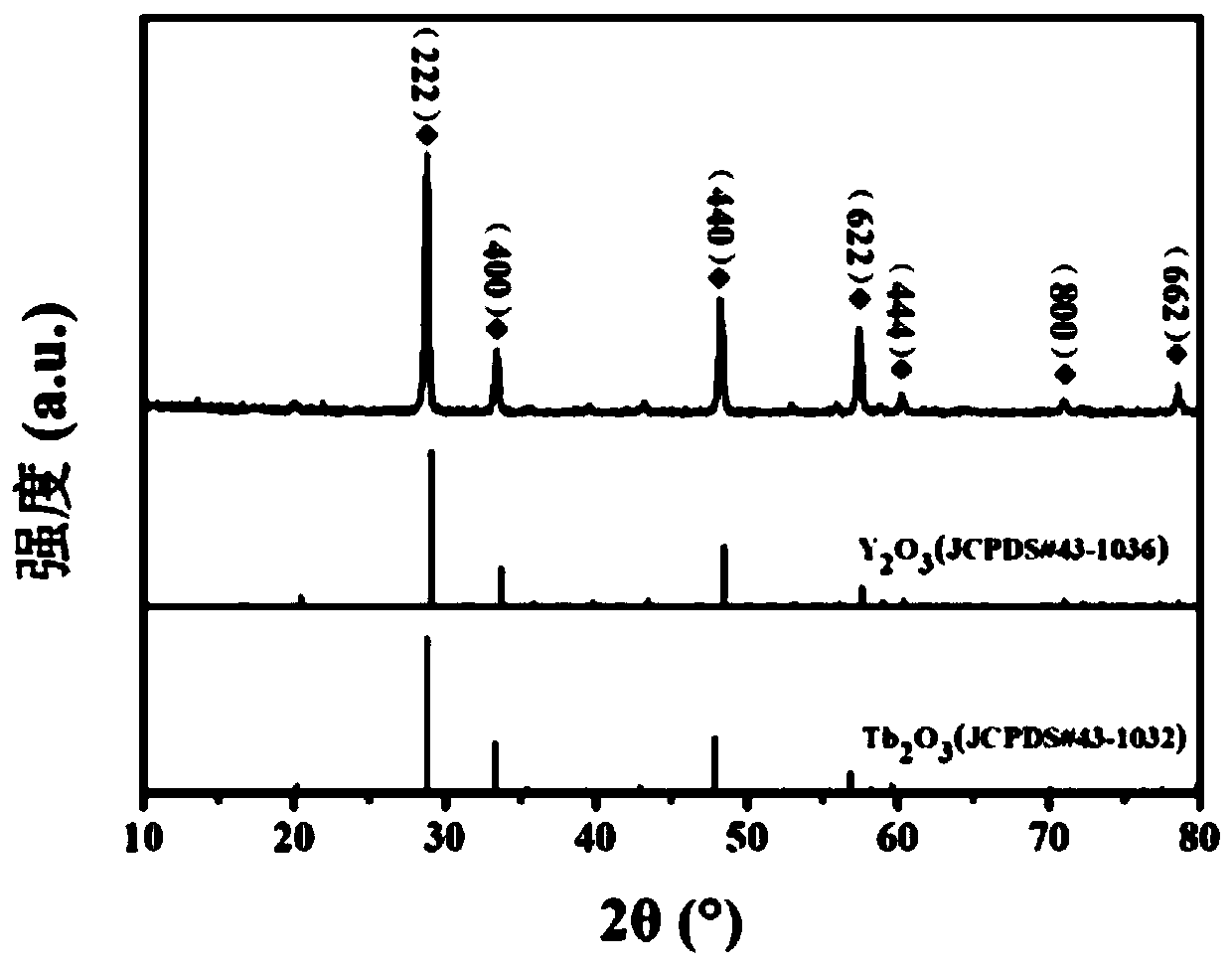
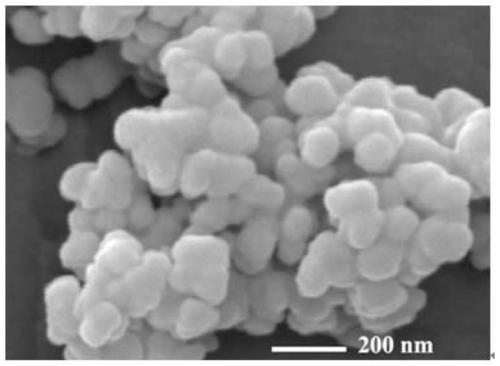
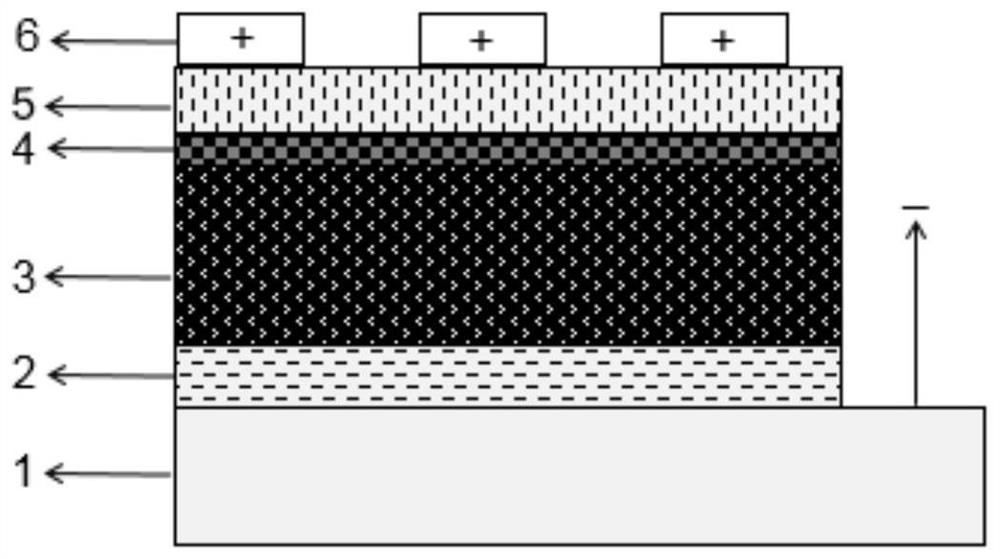
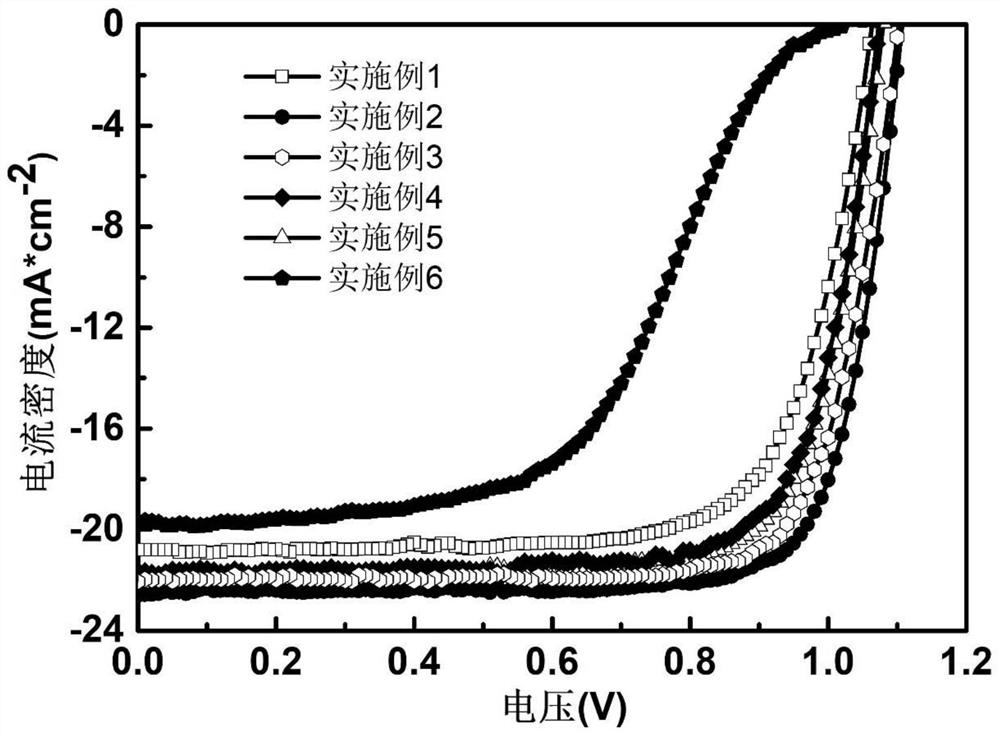
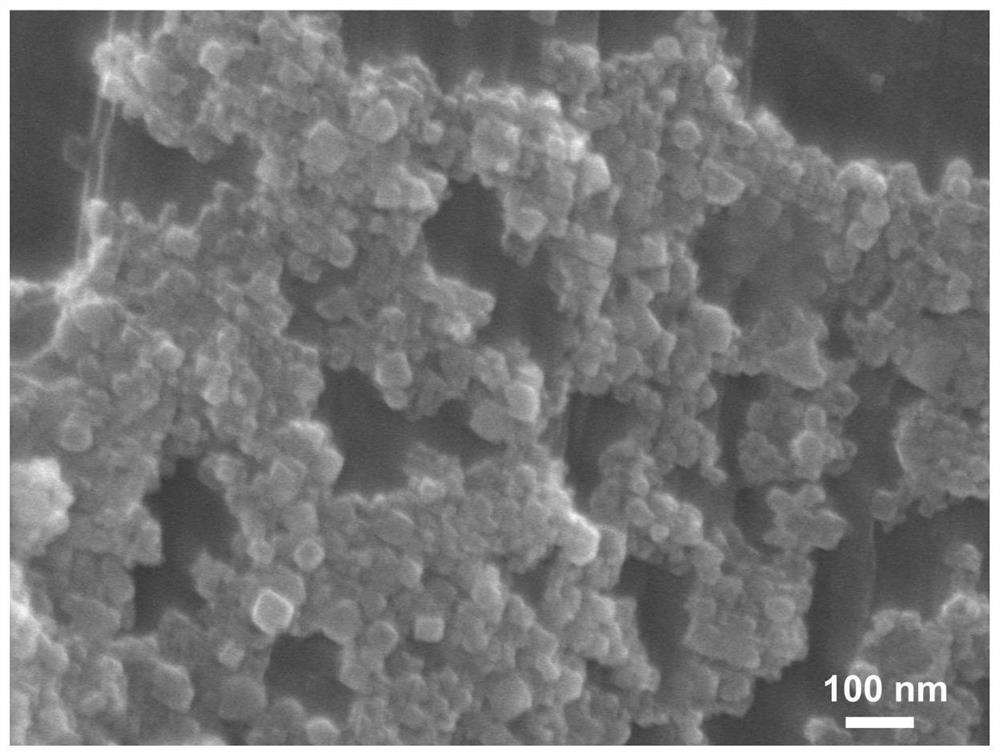
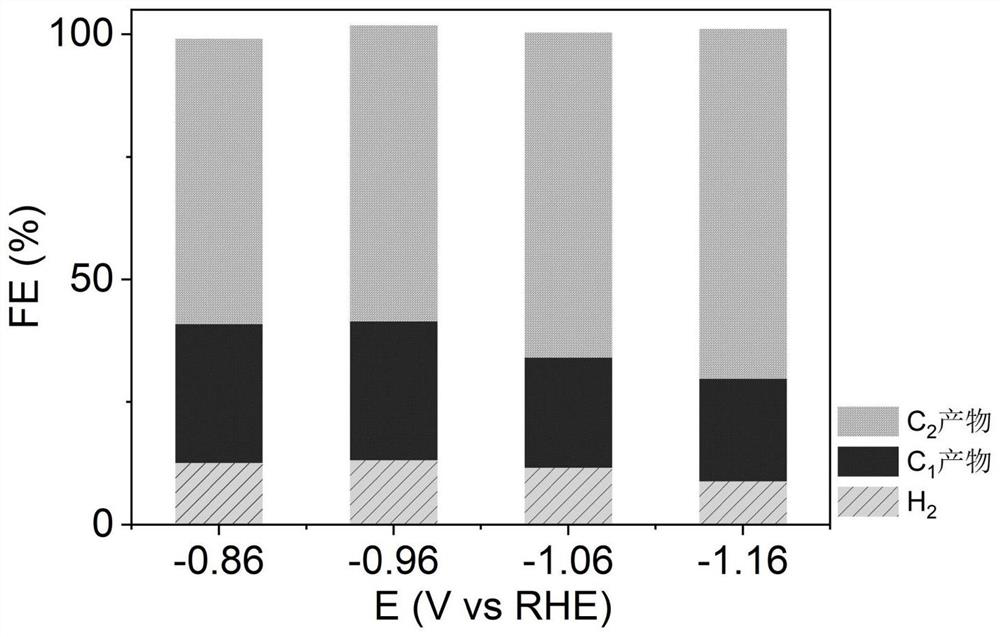
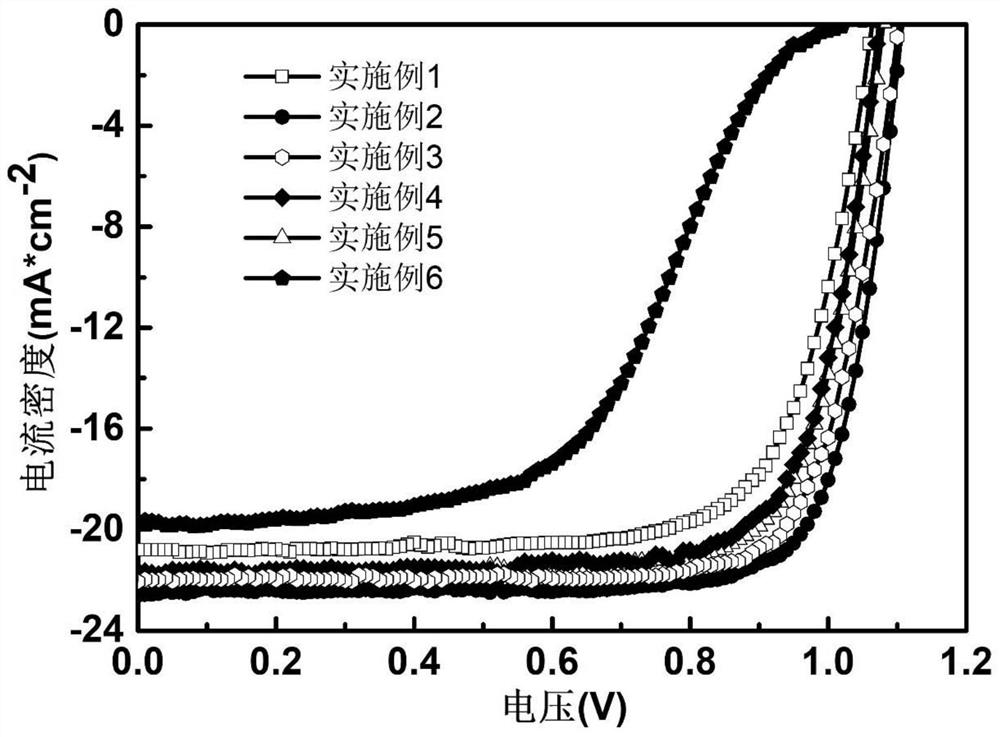
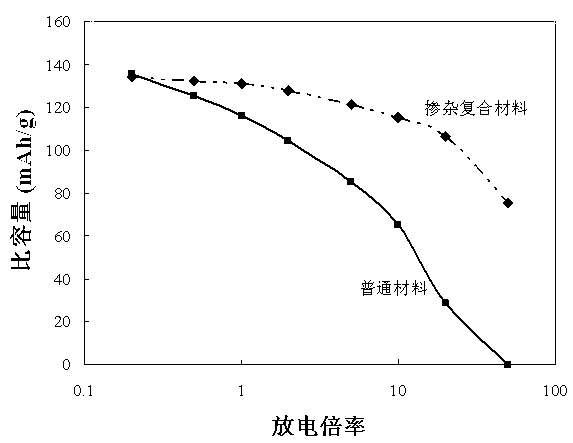
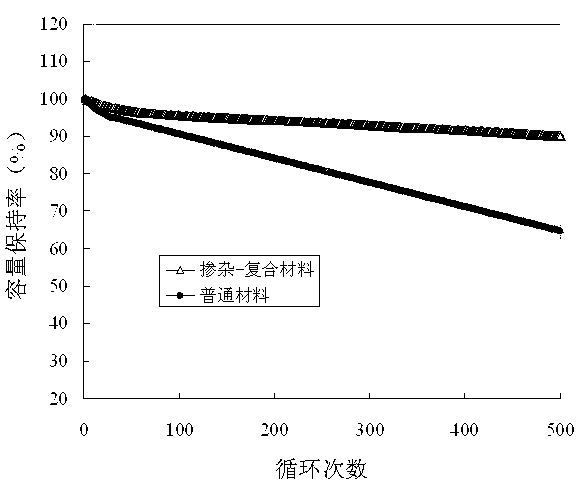
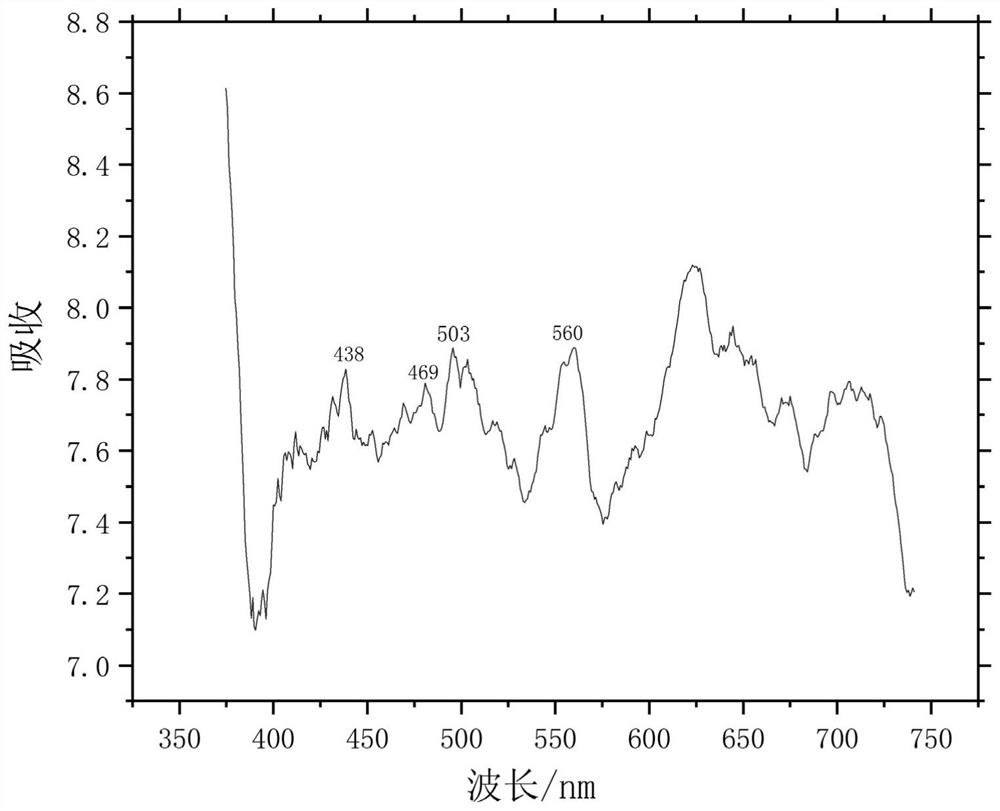
Patents
Literature
34results about How to "Valence stable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
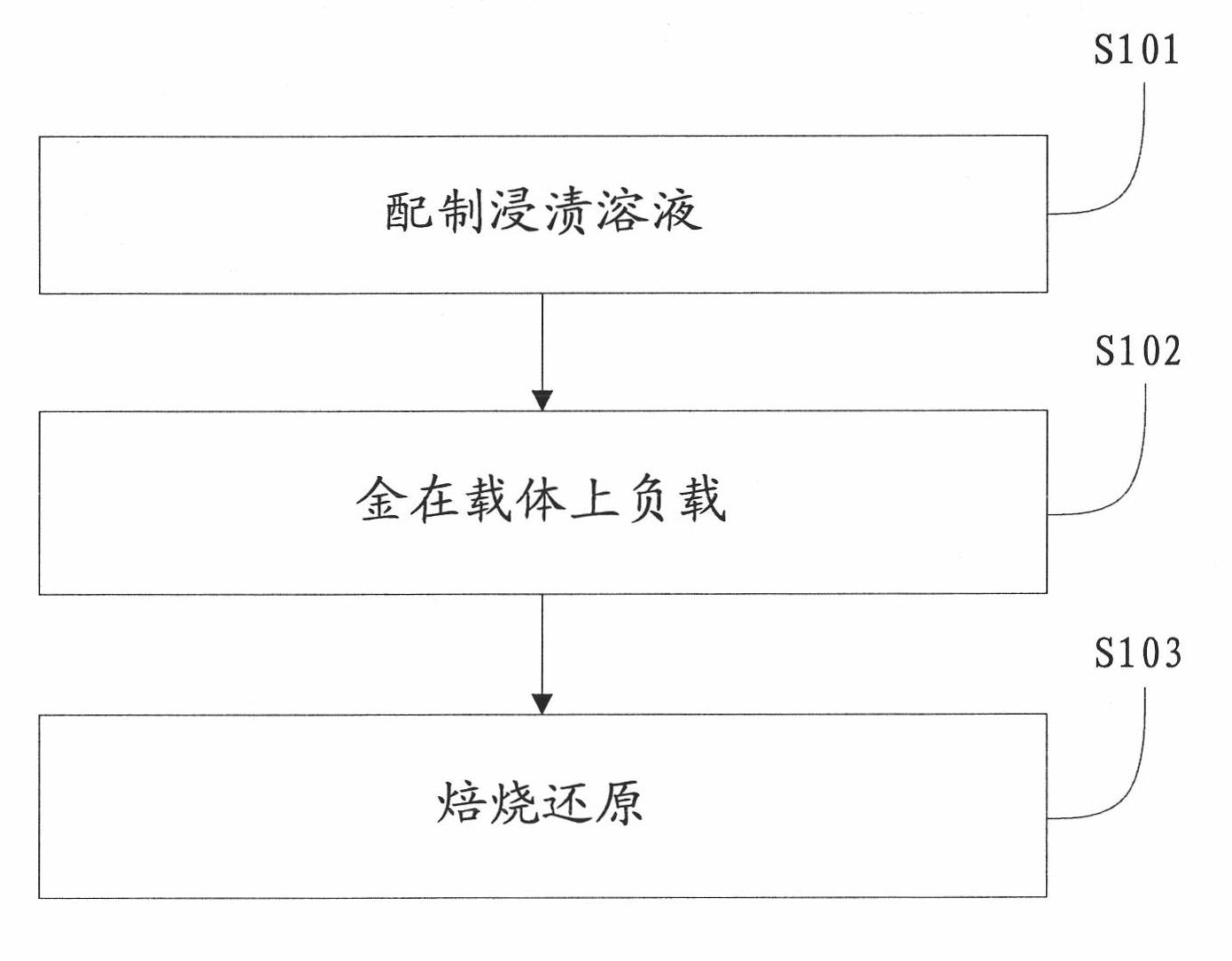
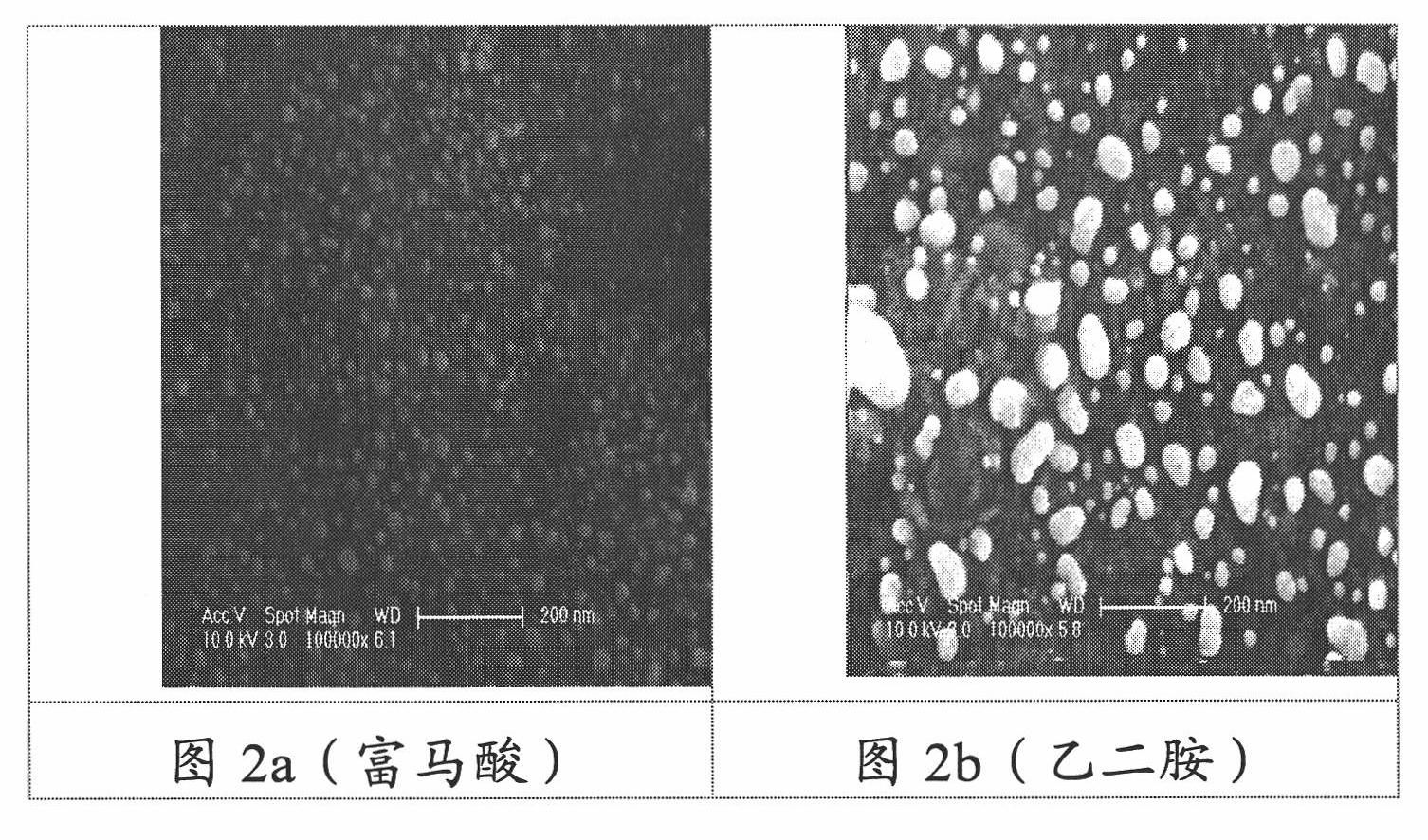
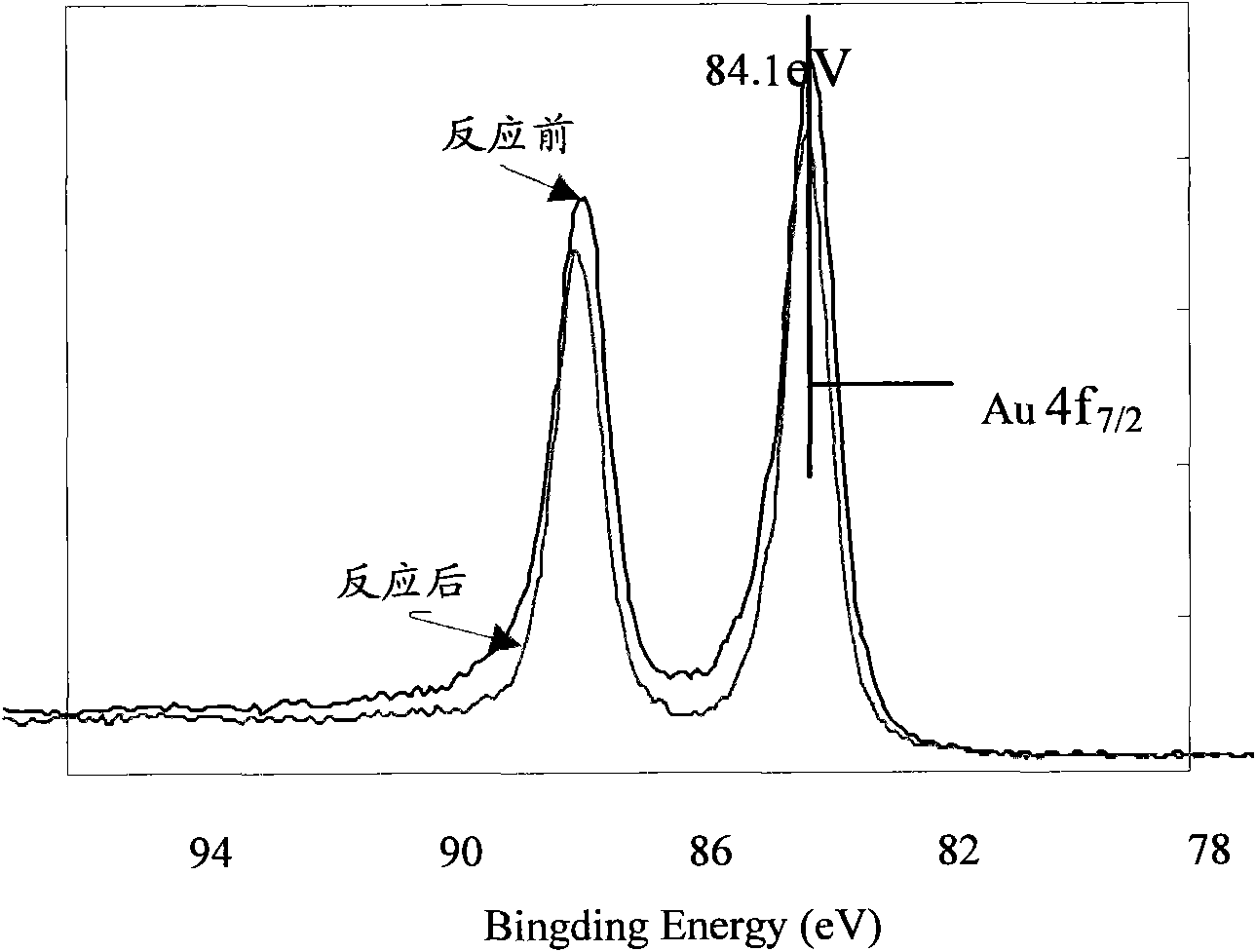
Method for preparing activated carbon-carried nano-gold catalyst
InactiveCN101785997AIncrease coverageValence stableDeodrantsMetal/metal-oxides/metal-hydroxide catalystsOrganic acidActivated carbon
The invention provides a method for preparing an activated carbon-carried nano-gold catalyst, comprising the following steps of preparing a dipping solution, adding at least one organic acid protective agent in the dipping solution, wherein the pKa value of the organic acid protective agent is close to the isoelectric point of the activated carbon / activated carbon fiber carrier, adjusting the pH value of the dipping solution to be close to the isoelectric point of the activated carbon / activated carbon fiber carrier; quickly and evenly putting the activated carbon / activated carbon fiber carrier in the dipping solution, mechanically stirring or ultrasonically mixing, standing, filtering, and then rinsing and drying the filtered carrier; roasting and reducing the dried gold-carried carrier to obtain an activated carbon / activated carbon fiber-carried nano-gold catalyst. The invention makes gold particles carried on the activated carbon have small size and be distributed evenly and improves the utilization rate of gold; and the low-concentration ozone and volatile organic compound in the air can be effectively dissolved by the catalyst.
Owner:TSINGHUA UNIV
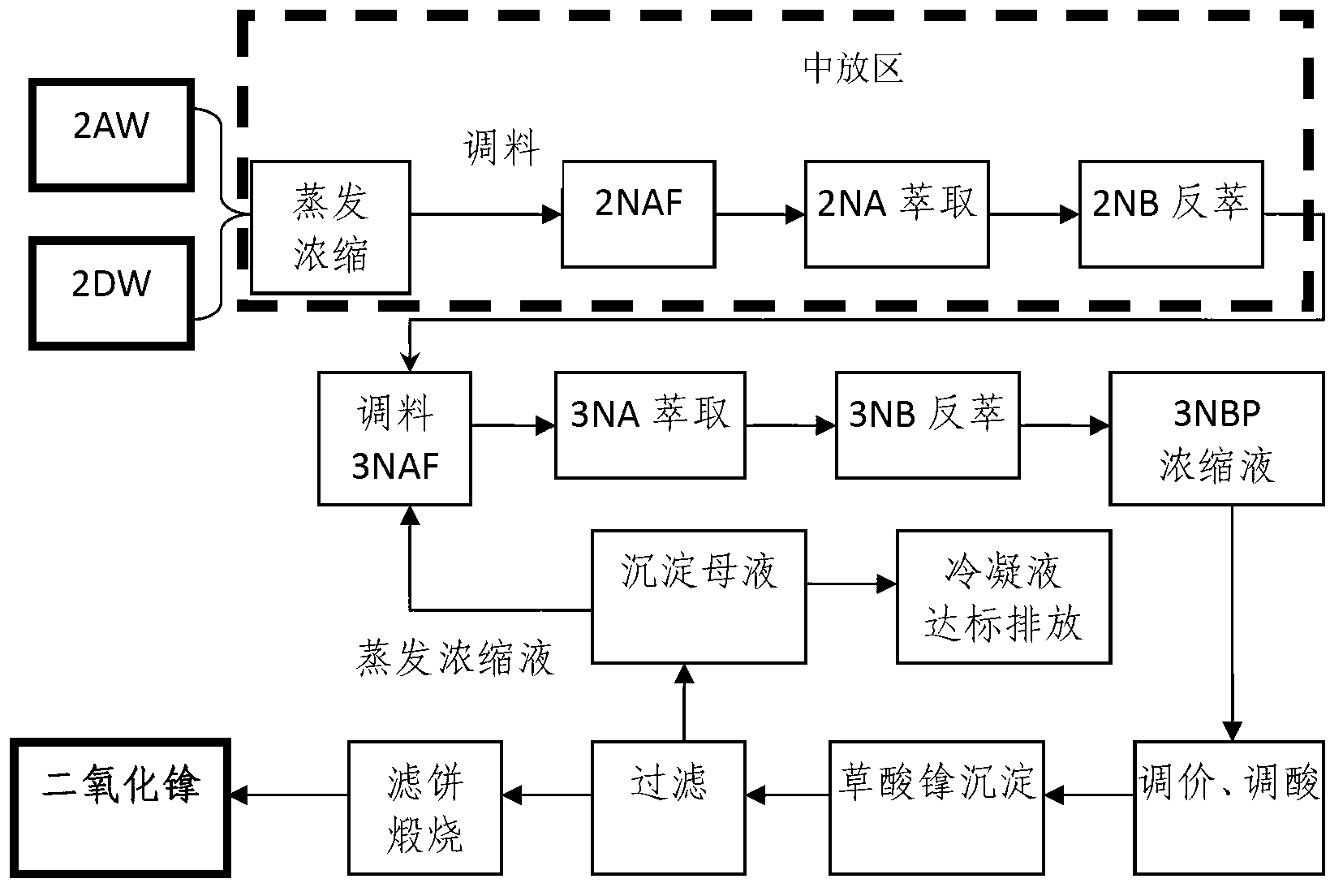
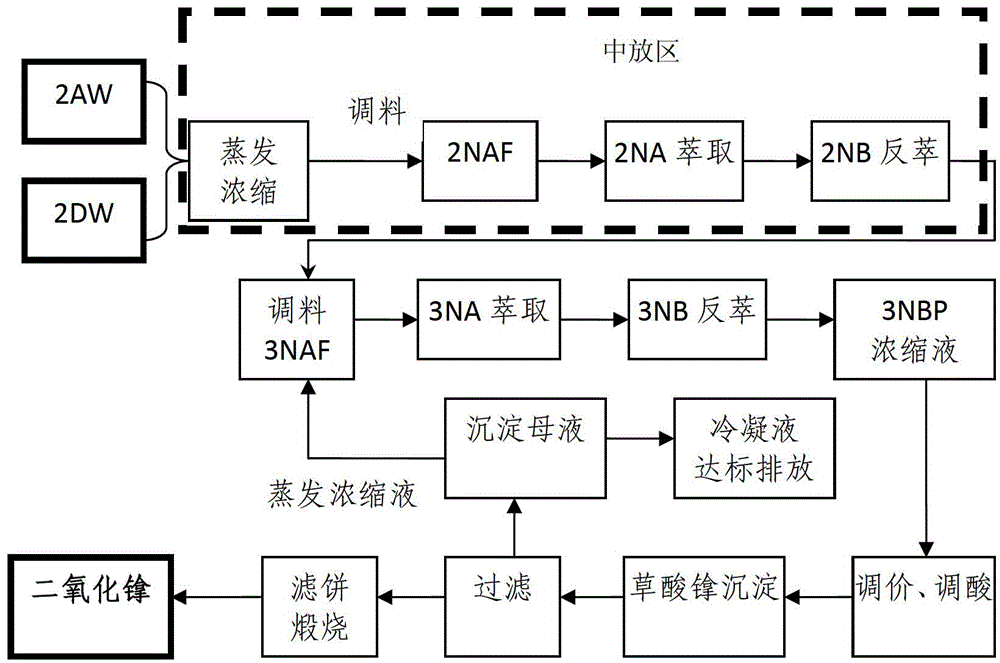
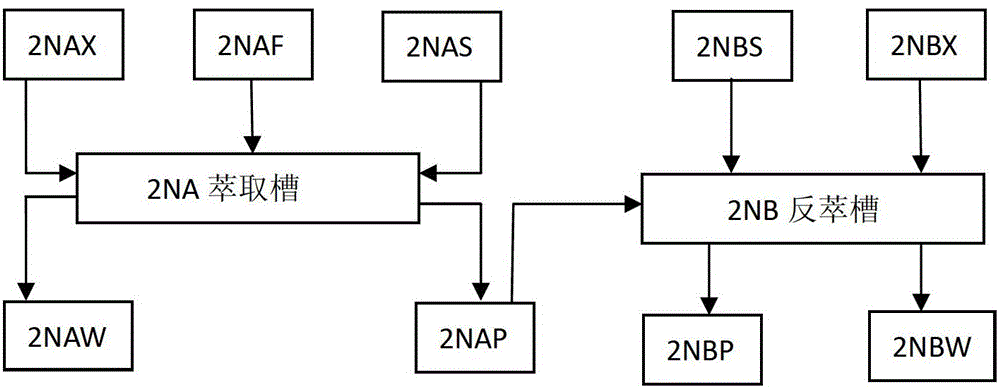
Process for recovering and purifying neptunium from waste liquor discharged from 2AW+2DW in Purex flow
ActiveCN103305702AHigh recovery rateValence stableProcess efficiency improvementRadioactive decontaminationOxalate precipitationEngineering
The invention belongs to the technical field of nuclear fuel reprocessing and discloses a process for recovering and purifying neptunium from waste liquor discharged from 2AW+2DW in Purex flow. The process comprises the primary neptunium purification process and the secondary neptunium purification process; the process comprises the following steps: by taking 30 volume percent of TBP-kerosene as an extraction agent, extracting Np, U and Pu to the organic phase, and allowing the fission fragments to enter the aqueous phase; adding a reducing agent and an extraction agent into the organic phase, reversely extracting the Np to the aqueous phase; further extracting uranium and plutonium, and performing oxalate precipitation, filtering and calcining on the obtained aqueous phase product to obtain the purified neptunium oxide solid. The process has the advantages that the neptunium recovery rate is over 98 percent, and the uranium and plutonium removal coefficient in neptunium is high.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
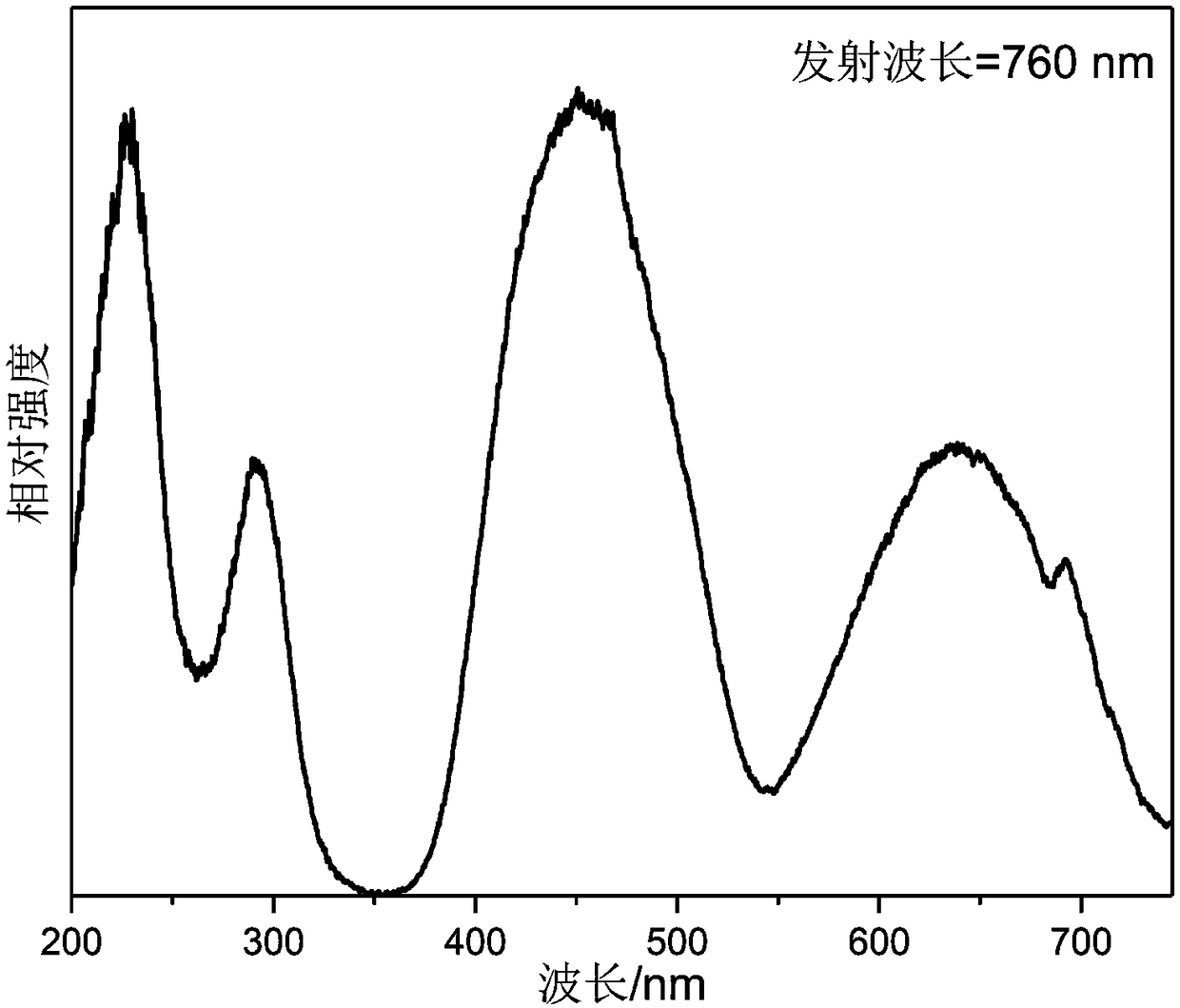
Near-infrared fluorescent powder with broadband emission characteristics, and preparation method and applications thereof
ActiveCN108424770APromote absorptionValence stableLuminescent compositionsSemiconductor devicesBroadbandTomography
The invention discloses a near-infrared fluorescent powder with broadband emission characteristics, and a preparation method and applications thereof, and belongs to the technical field of luminescentmaterial. The near-infrared fluorescent powder with broadband emission characteristics is suitable for near ultraviolet light and blue light chip excitation. The general chemical formula of the near-infrared fluorescent powder is Ca<2+x>Ln<1-x-y>Zr<2-x>Al<3>O<12>:xCr<3+>, yCe<3+>, and Cr<3+> is taken as a luminescence center, wherein Ln is used for representing one or a plurality of ions selectedfrom Y<3+>, Lu<3+>, and Gd<3+>, x and y are used for representing molar fractions, 0<x<=0.15, and 0<=y<=0.1. The near-infrared fluorescent powder can be taken as a light conversion material of near ultraviolet light LED chips or blue light LED chips, can be used for preparation of broadband near-infrared light sources, and is capable of satisfying requirements of blood oxygen detection or coherence tomography on infrared light sources with broadband emission characteristics.
Owner:CHANGCHUN INST OF OPTICS FINE MECHANICS & PHYSICS CHINESE ACAD OF SCI
Novel positive electrode material of lithium ion battery and preparation method of positive electrode material
InactiveCN105895909AAvoid destructionPromote circulationCell electrodesSecondary cellsLithium-ion batteryMaterials science
The invention relates to a novel positive electrode material of a lithium ion battery and a preparation method of the positive electrode material. The general formula of the positive electrode material is Li<y>(NiCoX<c>)O<2>, wherein X is an element more than or equal to +3 valence except Mn and comprises one or more of Ti, Zr, Ce, W, V, Cr, Sn, Sr, Mo, Sc, La, P, Nb, Y and Ga, y is more than or equal to 0.9 but less than or equal to 1.1, a is more than or equal to 0.3 but less than or equal to 0.8, b is more than or equal to 0.1 but less than or equal to 0.5, c is more than or equal to 0.01 but less than or equal to 0.3, (a+b+c) is equal to 1, c is less than or equal to a, and c is less than b. In the positive electrode material, the material damage caused by valence state change of the Mn is considered on the basis of the positive electrode material LiNiCoMnO<2> of the lithium ion battery, the Mn is substituted by the element X, the valence state of the element X is not changed, a good supporting and stabilizing effect can be exerted on a lattice structure, and thus, the cycle performance of the material is effectively enhanced.
Owner:QINGHAI TAIFENG XIANXING LITHIUM ENERGY TECH CO LTD
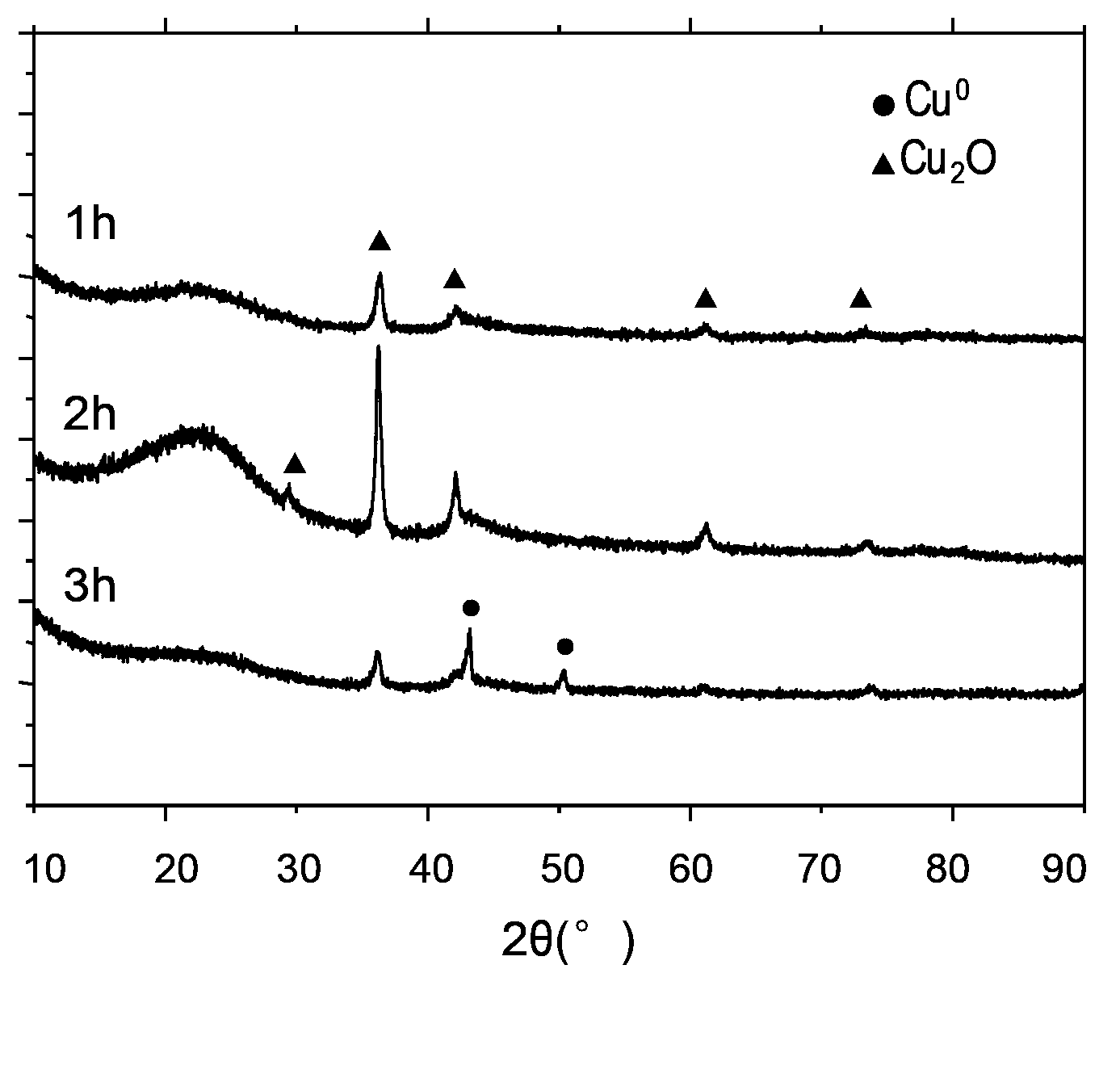
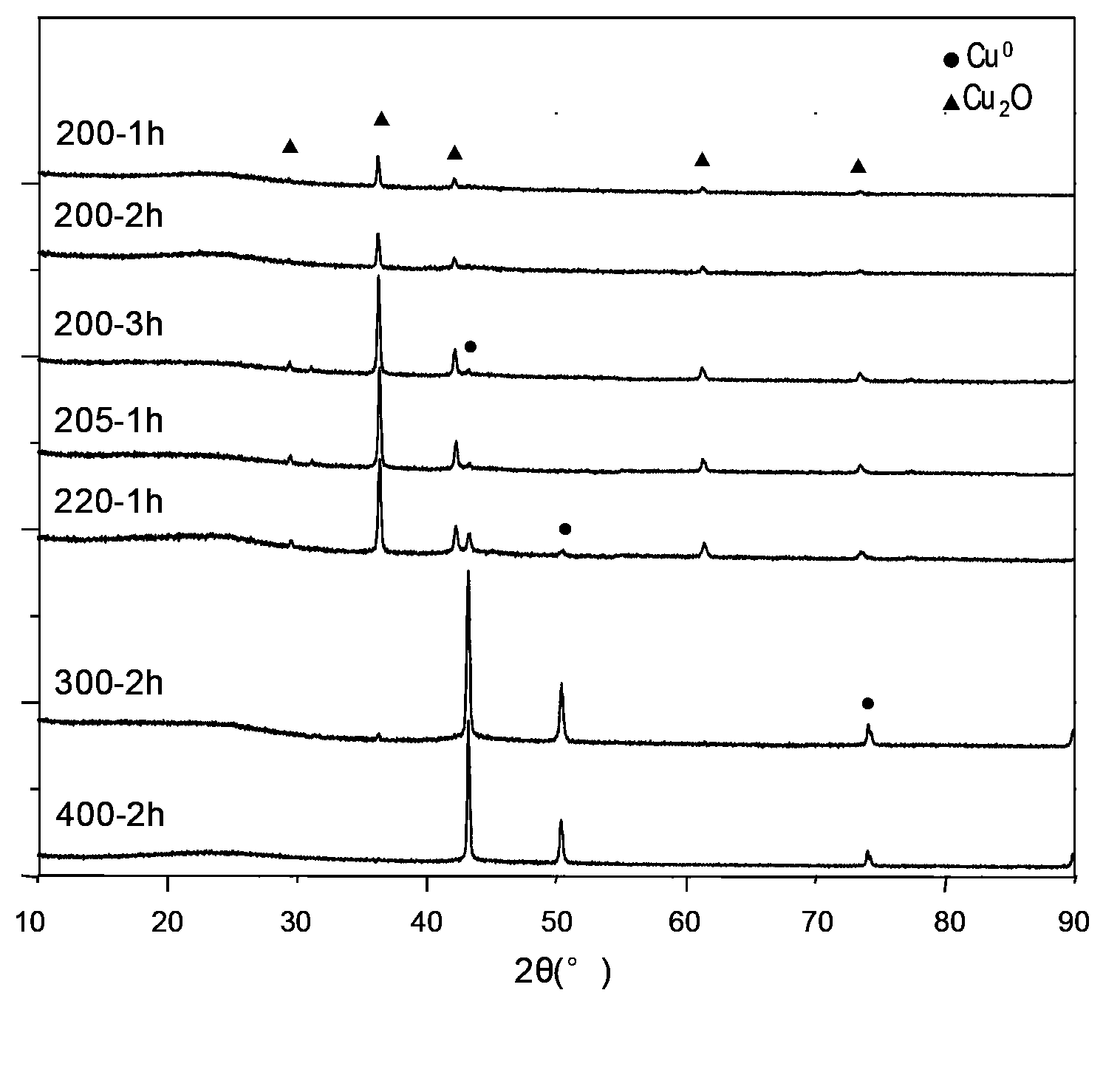
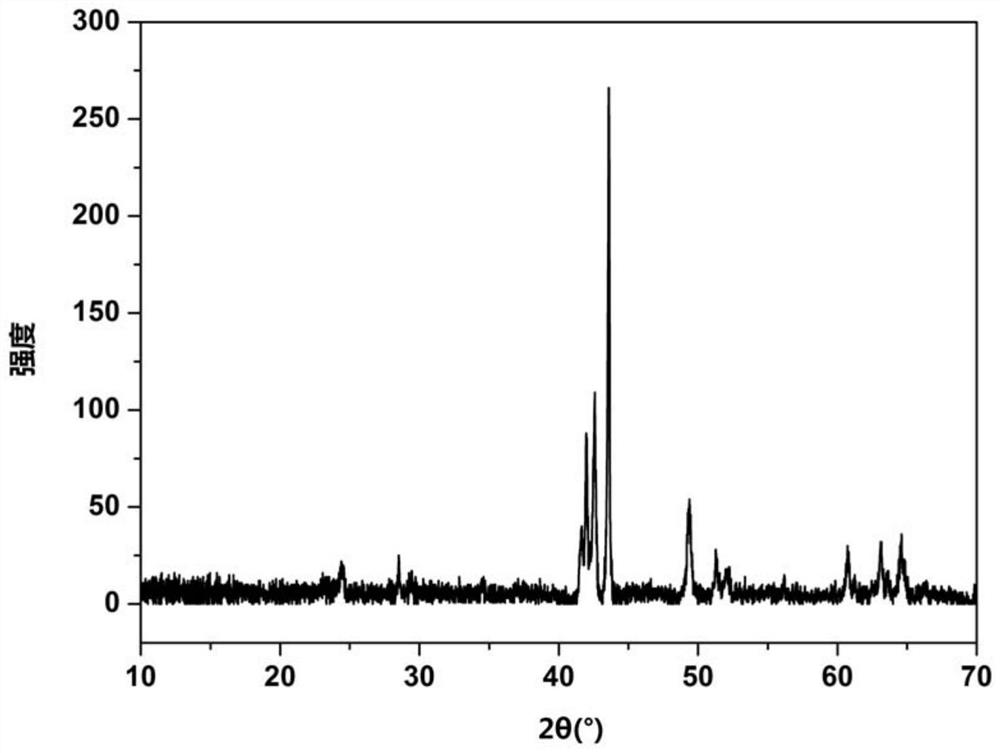
Activated carbon load single valence state cuprous oxide chloride-free catalyst, preparation method, and application in oxidate oxo synthesis dimethyl carbonate
InactiveCN103071497AImprove stabilityReduced activityOrganic compound preparationMetal/metal-oxides/metal-hydroxide catalystsActivated carbonChloride
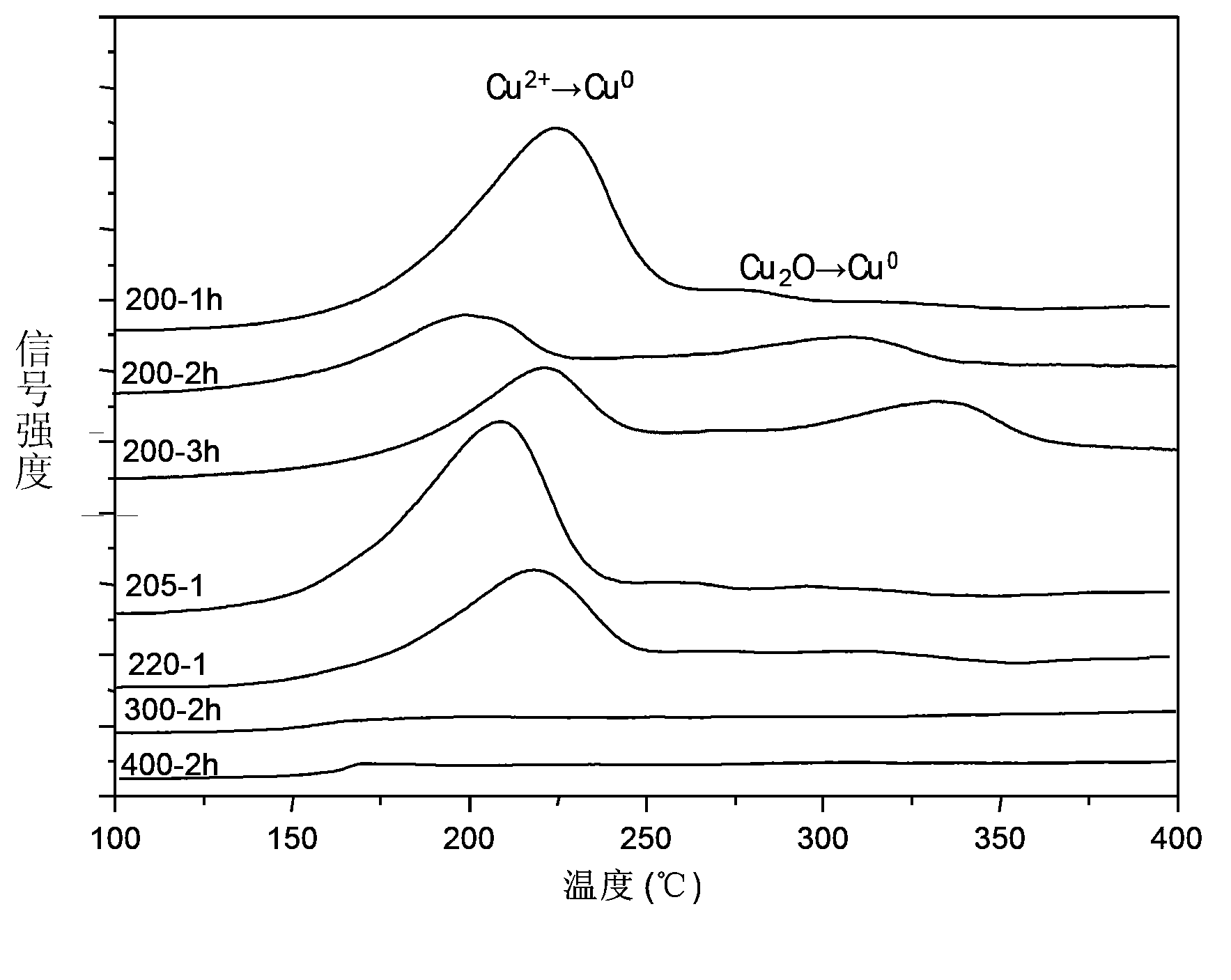
The invention provides an activated carbon load single valence state cuprous oxide chloride-free catalyst used for oxidating oxo synthesis carbonic ester, and a preparation method of the catalyst. The catalyst is a chloride-free catalyst taking activated carbon as a carrier; the active constituent of load is single valence state cuprous oxide, and the load capacity accounts for 12 to 22wt% of the carrier (copper is taken as the criteria). The preparation process comprises preparation of activated carbon load cupric acetate and preparation of activated carbon load single valence state cuprous oxide catalyst. The invention has the advantages that the preparation method of the catalyst is simple, the price is low, no chlorine exists when the catalyst provided by the invention is in use, the valence state of the active constituent is single and stable, sintering can not occur easily, and the product of dimethyl carbonate is high in selectivity, high in catalytic activity and the like.
Owner:TIANJIN UNIV
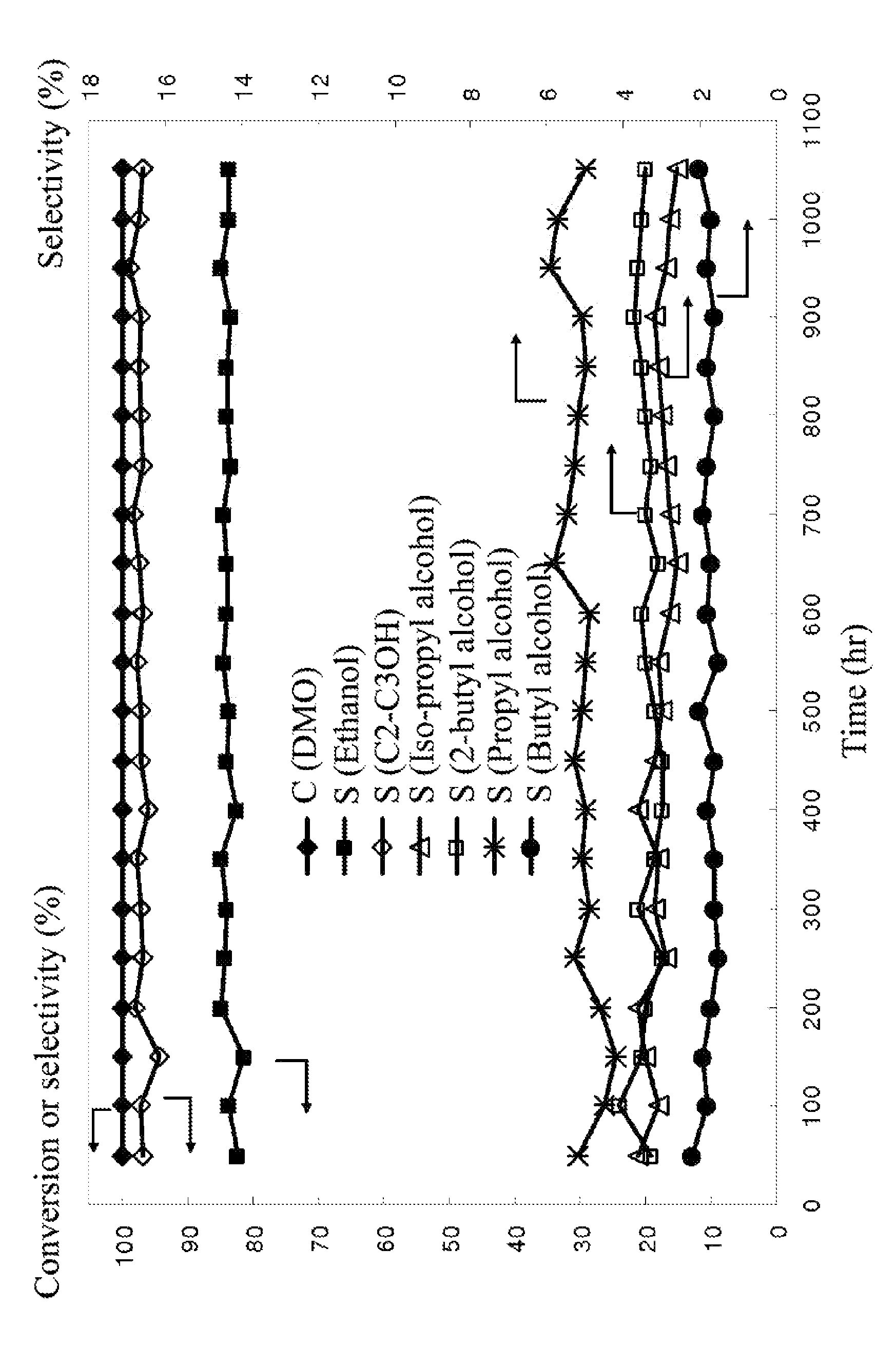
Catalyst for hydrogenation of oxalic ester to ethanol, method of preparing the catalyst, and method of using the same
ActiveUS20130261350A1Good dispersionImprove the immunityOrganic compound preparationOxygen compounds preparation by reductionCopperActive ingredient
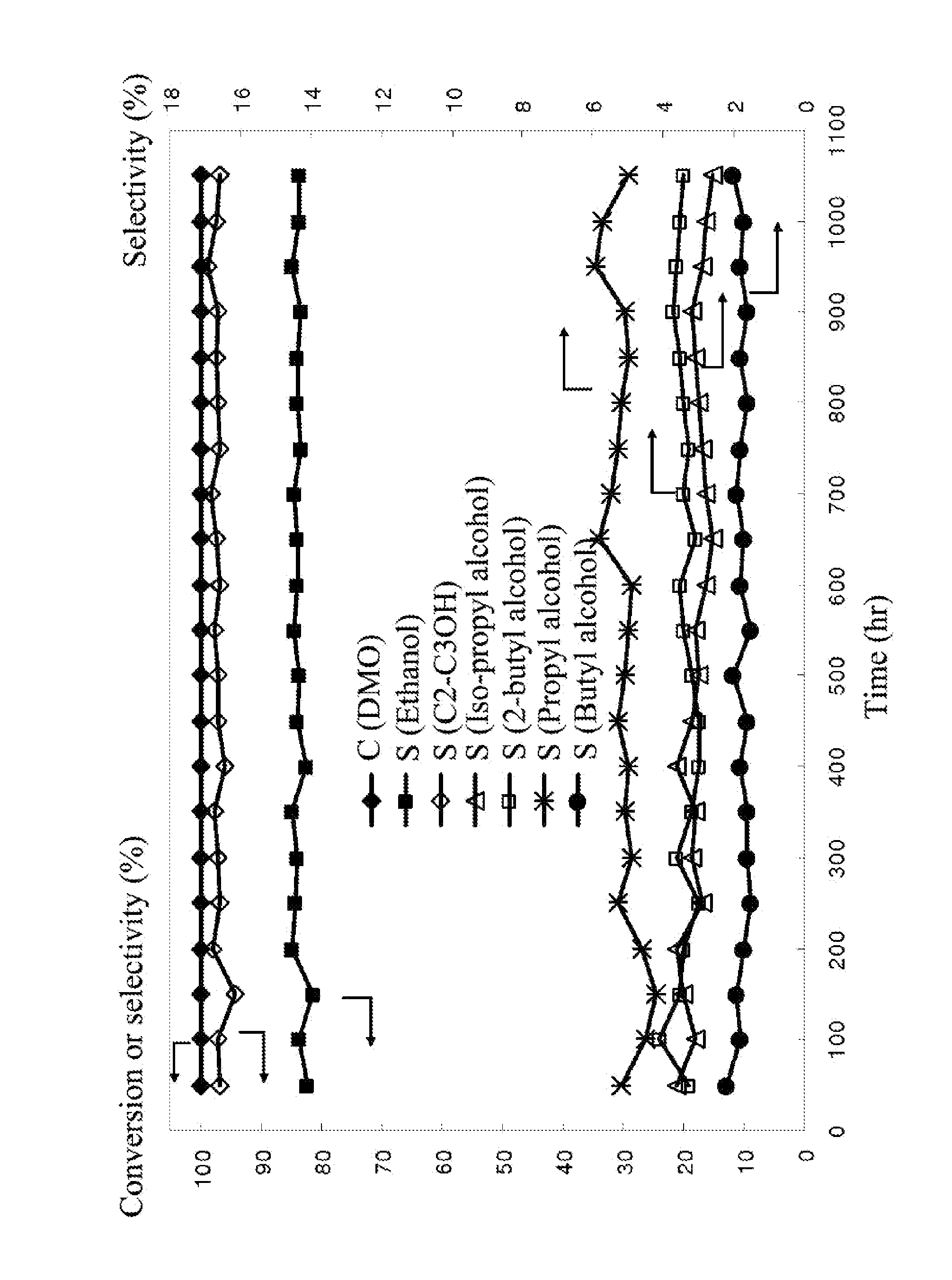
A catalyst including: a support, the support including a mixture of SiO2 and ZrO2; an active ingredient including copper; a first additive including a metal, an oxide thereof, or a combination thereof; and a second additive including Li, Na, K, or a combination thereof. The metal is Mg, Ca, Ba, Mn, Fe, Co, Zn, Mo, La, or Ce. Based on the total weight of the catalyst, the weight percentages of the different components are as follows: SiO2=50-90 wt. %; ZrO2=0.1-10 wt. %; copper=10-50 wt. %; the first additive=0.1-10 wt. %; and the second additive=0.1-5 wt. %.
Owner:TIANJIN UNIV
Preparation method of cobalt-coated lithium ion battery anode material
The invention discloses a preparation method of a cobalt-coated lithium ion battery anode material. The preparation method comprises the following steps of: coating Co(OH)2 on the surface of a lithium manganate matrix through chemical precipitation, wherein a complexing agent solution, a precipitant solution and a metal cobalt salt solution are added to a high-speed stirring reaction kettle, which is used for mixing up lithium manganate LiMn2O4 sizing agent, for carrying out a precipitation reaction; carrying out solid-liquid separating and drying into the discharged sizing agent after the cobalt precipitate is sufficiently reacted; carrying out high-temperature oxidation onto the materials by utilizing an oxidizing furnace under an oxygen atmosphere and a strong alkaline environment; carrying out pure water washing and solid-liquid separation onto the solid material after the oxidation reaction is completed; and obtaining the hydroxyl cobalt oxide-coated lithium manganate anode material after drying. The preparation method disclosed by the invention is low in device requirement, simple in process, good in material conductivity, long in cycle life, good in rate capability and high in capacity.
Owner:HUNAN SOUNDDON NEW ENERGY
Separation and enrichment determination method for Ir, Rh, Pt, Pd and Au in secondary resource material
InactiveCN105300961AThe method is simple and easyAddress gapsPreparing sample for investigationAnalysis by thermal excitationInductively coupled plasma emission spectroscopyMuffle furnace
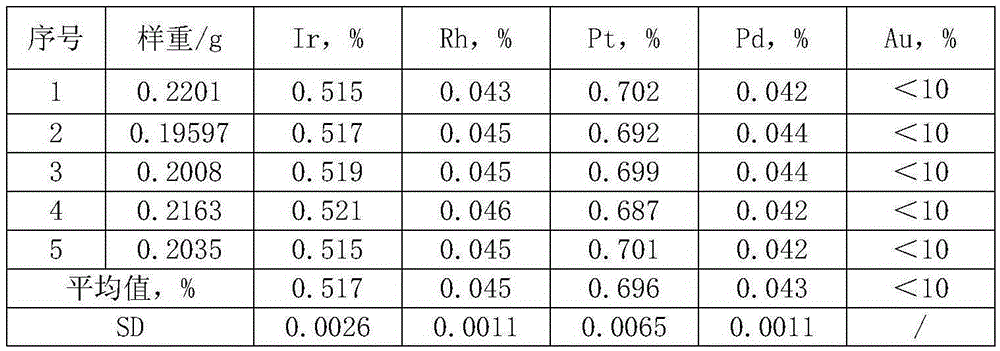
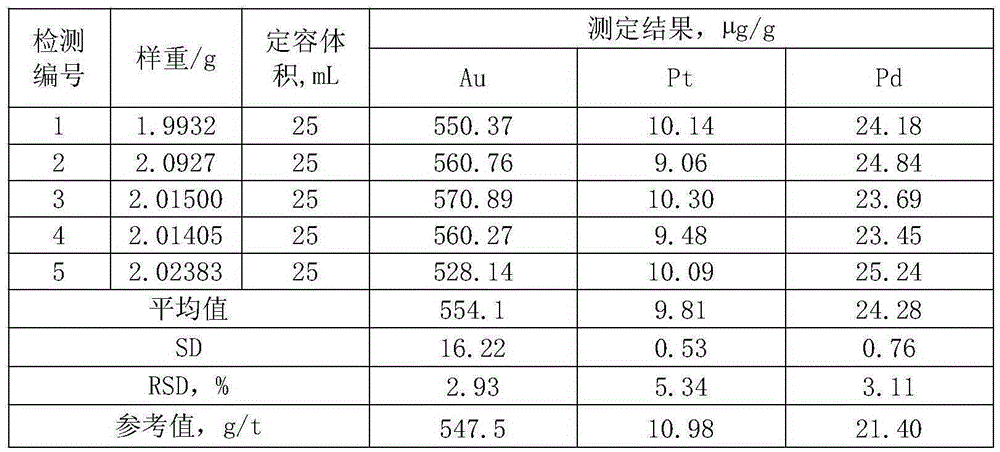
The invention discloses a separation and enrichment determination method for Ir, Rh, Pt, Pd and Au in a secondary resource material. The method includes the following steps that 1, samples are dissolved, and after high-temperature firing carbon removal, a Na2O2+NaCO3 mixed fluxing agent is adopted for fusion in a muffle furnace of 750 DEG C; 2, a test solution is oxidized, an appropriate quantity of sodium chlorate solution is added in a dissolving solution, boiling is performed, and Ir in the solution is oxidized so that the valence state can be made stable; the solution is adjusted to be at the appropriate volume, and the volume concentration of hydrochloric acid reaches 50%; 3, tellurium coprecipitation separation is carried out; 4, determination is carried out, an inductively coupled plasma optical emission spectrometry (ICP-AES) is used for determining the content of the Ir, Rh, Pt, Pd and Au in the solution obtained in the third step at the same time. The accurate and fast separation and enrichment determination method is specifically used for the Ir, Rh, Pt, Pd and Au with the content scope being 0.001%-5% in the secondary resource material such as waste and metallurgical middle materials formed in the using process of various precious metal products. The method is also suitable for separation and enrichment determination of Ir, Rh, Pt, Pd and Au single elements in the secondary resource material.
Owner:GUIYAN DETECTION TECH YUNNAN CO LTD
A kind of positive electrode material of lithium ion battery and preparation method thereof
InactiveCN102280618AHigh rate charge and discharge performance is goodIncrease migration rateCell electrodesFree coolingRoom temperature
The invention discloses a preparation method of an anode material of a lithium ion cell, comprising the following steps of: (1) uniformly dispersing a Li source, a Mn source, a Ni source, a doping element and a metal element with superionic conductor attributes to prepare a precursor, wherein the metal element with the superionic conductor attributes is selected from two or two more of Ti, La andZr; (2) drying or igniting the precursor, then carrying out pretreatment to obtain pretreatment powder; (3) pressing the pretreatment powder into a sheet; (4) carrying out heat treatment for 6-24h; and (5) annealing, naturally cooling to room temperature, grinding, and sieving to obtain the anode material. In the invention, on the one hand, a crystal skeleton of the material and a Mn valence state are stabilized by using the doping element, on the other hand, a compound lithium ion superionic conductor improves a migration rate of the lithium ion in the material and improves the multiplying power property and cycling property of an electrode are improved.
Owner:SUZHOU UNIV
Ammonium ferrous phosphate as well as preparation method and application thereof
ActiveCN111115608AThe preparation method is reasonableStable lattice structureCell electrodesPhosphorus compoundsFerrous ammonium phosphateFerrous salts
The invention provides ammonium ferrous phosphate as well as a preparation method and application thereof, the general formula of the ammonium ferrous phosphate is NH4FexM(1-x)PO4, and x is greater than 0 and less than or equal to 1; and M is selected from one or more of V, K, Co, Mn, Zn, Mg, Ni, Al, Ti, Nb, Zr and Cu. The invention also discloses a method for preparing lithium iron phosphate fromthe ammonium ferrous phosphate, and the method comprises the following steps: mixing ferrite, a phosphorus source and ammonia water, regulating the pH value of the reaction system to 6-7, and reacting to generate the ammonium ferrous phosphate, wherein the molar ratio of the ferrite to the phosphorus source is (0.95-1.03): (0.95-1.03); reducing and calcining the ammonium ferrous phosphate at hightemperature under inert atmosphere protection to obtain ferrous pyrophosphate; mixing the ferrous pyrophosphate with lithium salt and a carbon source, crushing and ball-milling; performing calciningand coating carbon, and synthesizing to obtain the lithium iron phosphate. The method is simple in production process, low in production precision requirement and easy to prepare and dope.
Owner:DALIAN RONGKE ENERGY STORAGE GRP CO LTD
Method for regulating and controlling interaction between metal nanoparticles and carrier by plasma
ActiveCN112275284AHigh reproducibility of preparationEasy to makeHydrogenHydrocarbon by hydrogenationNano catalystPtru catalyst
The invention discloses a method for regulating and controlling interaction between metal nanoparticles and a carrier by plasma, which comprises the following steps: S1, loading a metal precursor on the carrier by adopting an impregnation method or a deposition-precipitation method, and cleaning and drying to obtain a catalyst; s2, putting the obtained catalyst into a plasma generating device, andpretreating the catalyst by adopting plasma in a reducing atmosphere; s3, in an oxidizing atmosphere, activating the catalyst by adopting plasma; and S4, after activation treatment, treating the catalyst by adopting plasma in a reducing atmosphere to obtain the supported metal catalyst. According to the method disclosed by the invention, the supported catalyst is pretreated, activated and retreated in sequence by utilizing plasmas generated by discharging in different atmospheres, so that the interaction between the metal nanoparticles and the carrier is effectively modulated, and the stability of the nano-catalyst is remarkably enhanced while the sizes of the metal nanoparticles are controlled.
Owner:DALIAN MARITIME UNIVERSITY
Yttria-stabilized terbia powder, magneto-optic transparent ceramic and preparation method of magneto-optic transparent ceramic
The invention provides an yttria-stabilized terbia powder, a magneto-optic transparent ceramic and a preparation method of the magneto-optic transparent ceramic. The preparation method of the yttria-stabilized terbia magneto-optic transparent ceramic is characterized by preparing yttria-stabilized terbia nanopowder through a wet chemistry coprecipitation method, preparing a ceramic biscuit from the yttria-stabilized terbia nanopowder, and performing pressureless sintering at 1600-1800 DEG C in a reducing atmosphere to obtain the yttria-stabilized terbia magneto-optic transparent ceramic, wherein the structural formula of the yttria-stabilized terbia magneto-optic transparent ceramic is (TbxY1-x)2O3, and 0<=x<=1. The yttria-stabilized terbia magneto-optic transparent ceramic is less prone to cracking during sintering, good in optical transmittance and high in Verdet constant.
Owner:SHANGHAI INST OF TECH
Preparation method and application for vanadium trioxide negative electrode material
ActiveCN108557885AHigh specific capacityAvoid contactSecondary cellsNegative electrodesLevel structureLithium-ion battery
The invention belongs to the technical field of lithium ion batteries, and particularly relates to a preparation method and application for a vanadium trioxide negative electrode material. The preparation method for the vanadium trioxide negative electrode material comprises the steps that an ammonium vanadate compound is taken as a precusor substance, a silicon wafer is taken as a carrier, a lithium sheet is taken as a reducing agent, the above substances are placed in a crucible and is calcined in a tube furnace, the above substance is cooled to the indoor temperature naturally, and a V2O3 negative material is obtained. The preparation method is simple and feasible, the production cost is low, and the safety coefficient is high; the prepared V2O3 negative material is of a multi-level structure, and material shape can be controlled. In addition, the prepared V2O3 negative material is used for assembling a half cell, the result shows that the V2O3 negative material is high in specificcapacity, good in rate capability and stable in circular performance. The prepared V2O3 material is taken as the negative material to produce lithium ion batteries and has a wide application prospect.
Owner:JIANGSU UNIV
Perovskite photovoltaic cell and preparation method thereof
ActiveCN111710781AHigh cost of preparationImprove crystal qualitySolid-state devicesSemiconductor/solid-state device manufacturingPerovskite solar cellPerovskite (structure)
The invention relates to a high-performance perovskite cell and a preparation method thereof. The perovskite solar cell uses a Fe2-xMgxO3 thin film prepared by a solution method as an interface passivation layer to passivate adjacent interfaces of a perovskite photosensitive layer of a perovskite photovoltaic cell, x is greater than or equal to 0 and less than or equal to 0.2, and the thickness is30-40 nm. According to the perovskite photovoltaic cell, Fe2-xMgxO3 is used as an interface passivation layer, the preparation cost of the perovskite cell is greatly reduced, the crystallization quality of a perovskite thin film is improved by utilizing the interaction of iron, iodine, oxygen and lead, meanwhile, carriers can be rapidly migrated by the Fe2-xMgxO3 thin film, and charge accumulation at an interface is reduced; a proper amount of magnesium element can also form strong chemical bonds with surrounding ions, the structure of adjacent interfaces of perovskite is stabilized, interface defects are reduced, non-radiative recombination is reduced, and the purpose of improving device performance is improved.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Preparation method and application of nano-copper catalyst
ActiveCN114277398AEasy to prepareMild conditionsPhotography auxillary processesElectrolytic organic productionOrganic acidPtru catalyst
The invention discloses a preparation method and application of a nano-copper catalyst. The method comprises the following steps: (1) preparing a bicarbonate solution, and dissolving an organic acid salt and a copper salt precursor in the bicarbonate solution; and (2) placing a conductive substrate in the solution obtained in the step (1), introducing at least one of N2 and CO2 gases, and simultaneously performing electrochemical reduction to obtain the nano-copper catalyst. The preparation method of the catalyst is simple, mild in condition and easy to operate. In the electrochemical reduction preparation process of the copper catalyst, the organic anions can be coordinated with copper on the surface of the catalyst and are induced to form an oriented crystal face, so that the stability and activity of the nano-copper catalyst are improved. When the nano-copper catalyst is applied to the process of electrocatalytic reduction of CO2, organic anions coordinated on the surface of the nano-copper catalyst can stabilize the valence state of surface copper and inhibit structural recombination of the catalyst in the electrocatalytic process, so that the nano-copper catalyst has excellent electrocatalytic reduction performance and stability of CO2.
Owner:BEIHANG UNIV
Chemical oil removal liquid suitable for aluminum alloy by utilizing anode oxidation waste sulfuric acid and application of chemical oil removal liquid
InactiveCN108193213AImprove wettabilityReduce adhesionSulfur-trioxide/sulfuric-acidEutrophicationAcid washing
The invention discloses a chemical oil removal liquid suitable for aluminum alloy by utilizing anode oxidation waste sulfuric acid and an application of the chemical oil removal liquid. The oil removal liquid is composed of sulfuric acid, an emulsifier, a nonionic surfactant, an anionic surfactant, a builder, an oxidizing agent and a corrosion inhibitor. According to the chemical oil removal liquid, the waste sulfuric acid solution of the anodic oxidation production line is fully utilized to prepare the oil removal liquid medicine, the reutilization of the anode oxidation waste acid is realized, and the cost for producing acid washing oil removal and anode oxidation is saved. The oil removal liquid is free of phosphorus, nitrogen and the like, the wastewater treatment is simple, the eutrophication of the water body can be avoided after the water is discharged, and the environment-friendly performance is good.
Owner:SHENYANG OUSHIDUN NEW MATERIAL TECH
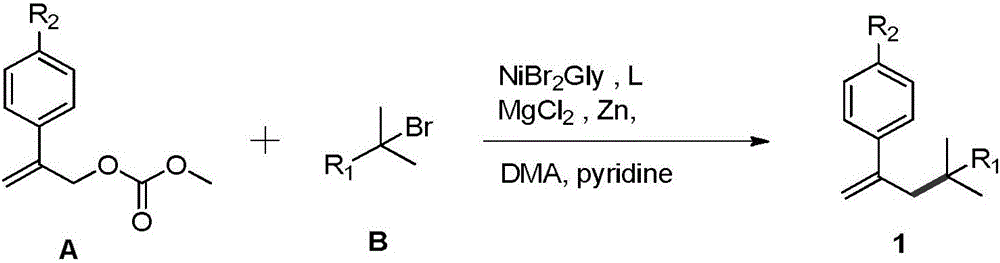
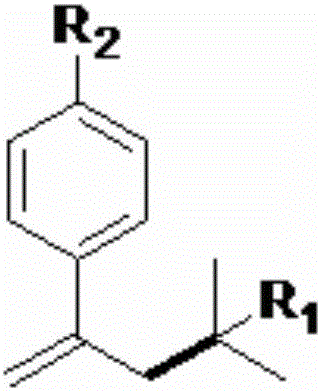
Styrenic quaternary carbon compound and preparation method thereof
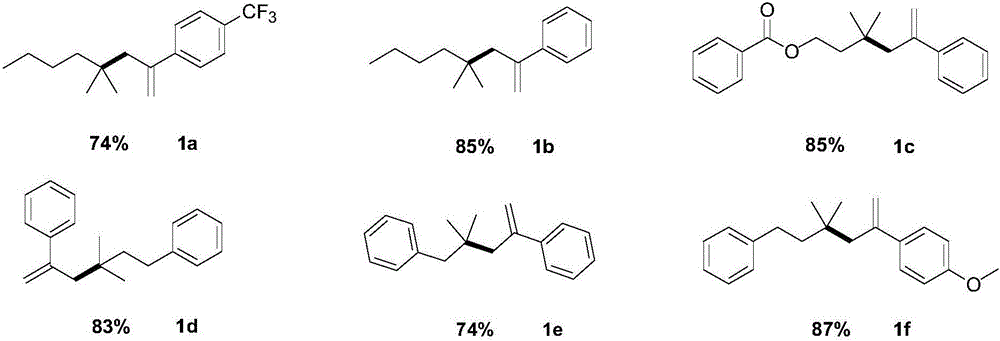
ActiveCN106588651ALow priceValence stableOrganic compound preparationOrganic chemistry methodsStructural formulaReducing agent
The invention relates to a styrenic quaternary carbon compound and a preparation method thereof. The general structural formula of the styrenic quaternary carbon compound is (img file='dest_path_image002. TIF'wi='86'he='122' / ), wherein R1 is C1-C8 alkyl chain or aromatic group and R2 is the substituent group of OMe and CF3. The reaction of the styrenic quaternary carbon compound and the preparation method thereof is the catalytic-reduction coupled reaction of metallic nickel, wherein the reducing agent is the environmentally friendly Zn powder and the catalyst is nickel bromide glycol dimethyl ether (NiBr2. glyme) which has the advantage of low cost and stable valence. The reaction condition has the advantages of being mild, being simple to operate, having good acceptability for functional groups and higher productivity as well as producing no-isomerized by-products of tertiary halogenated substrates.
Owner:SHANGHAI UNIV
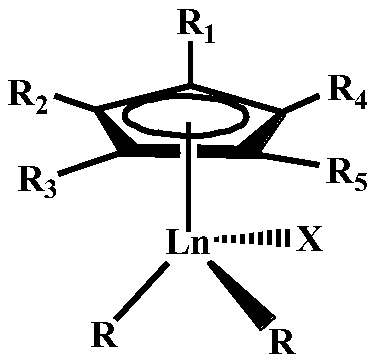
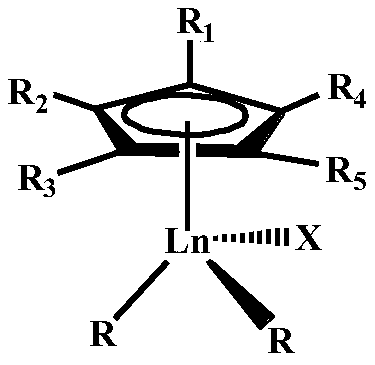
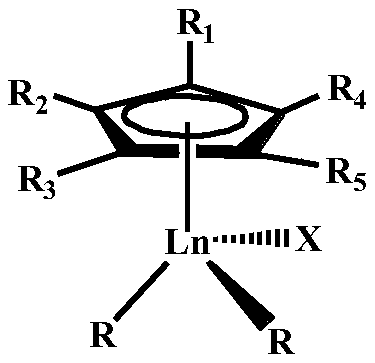
Star-branched ternary integrated rubber prepared through catalysis of mono-scandium-cyclopentadienyl rare earth catalyst and preparation method of star-branched ternary integrated rubber
ActiveCN114195947AValence stableEasy to prepareRolling resistance optimizationComposite materialDouble bond
The invention belongs to the technical field of functional high polymer materials, and provides star-branched ternary integrated rubber prepared through catalysis of a mono-scandium-cyclopentadienyl rare earth catalyst and a preparation method of the star-branched ternary integrated rubber. The star-branched ternary integrated rubber is prepared by copolymerizing a macromonomer containing a plurality of overhanging double bonds, amino-containing styrene, isoprene and butadiene under the catalysis of a mono-scandium-cyclopentadienyl rare earth catalyst. The content of amino-containing styrene is 5%-50% by mole, and the content of isoprene is 20%-90% by mole; the content of the 3, 4-polyisoprene is 3%-78% based on the total molar amount of the polyisoprene being 100%; the content of the 1, 4-polybutadiene is 50%-98% based on 100% of the total mole of the polybutadiene; the amino-containing styrene is selected from styrene derivatives containing nitrogen substituent groups. The prepared star-branched terpolymer can effectively improve the blending performance of the star-branched terpolymer and a polar filler, increase the acting force between a rubber matrix and the filler, and improve the physical and mechanical properties of a rubber material.
Owner:DALIAN UNIV OF TECH
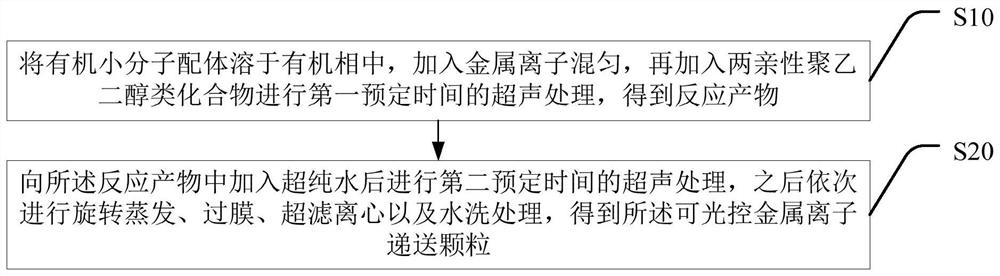
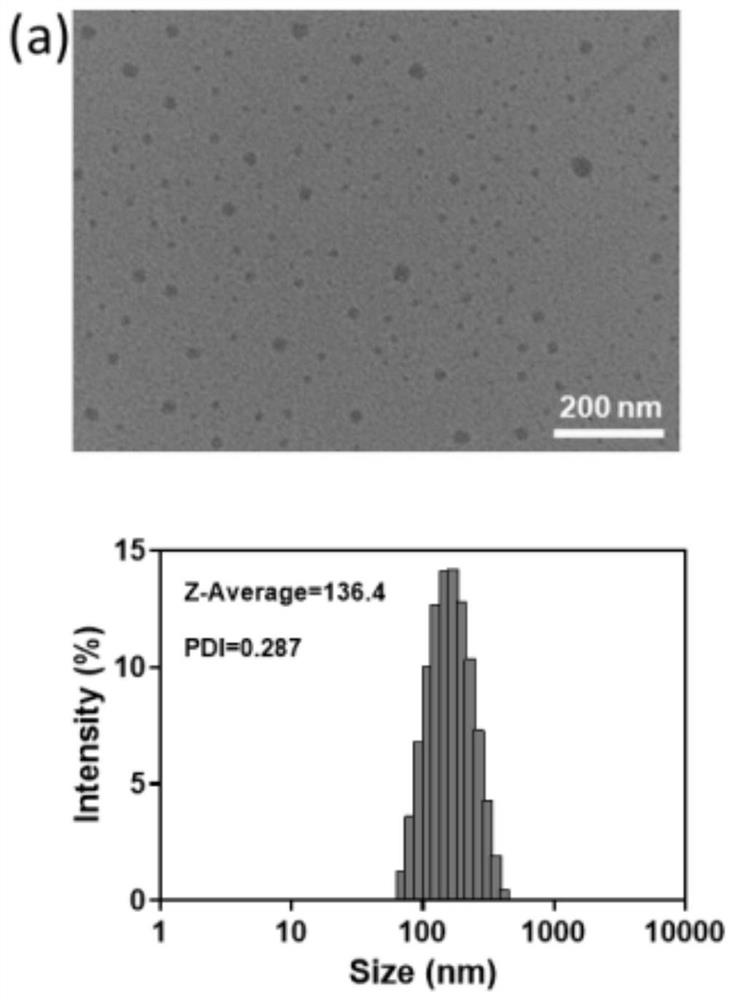
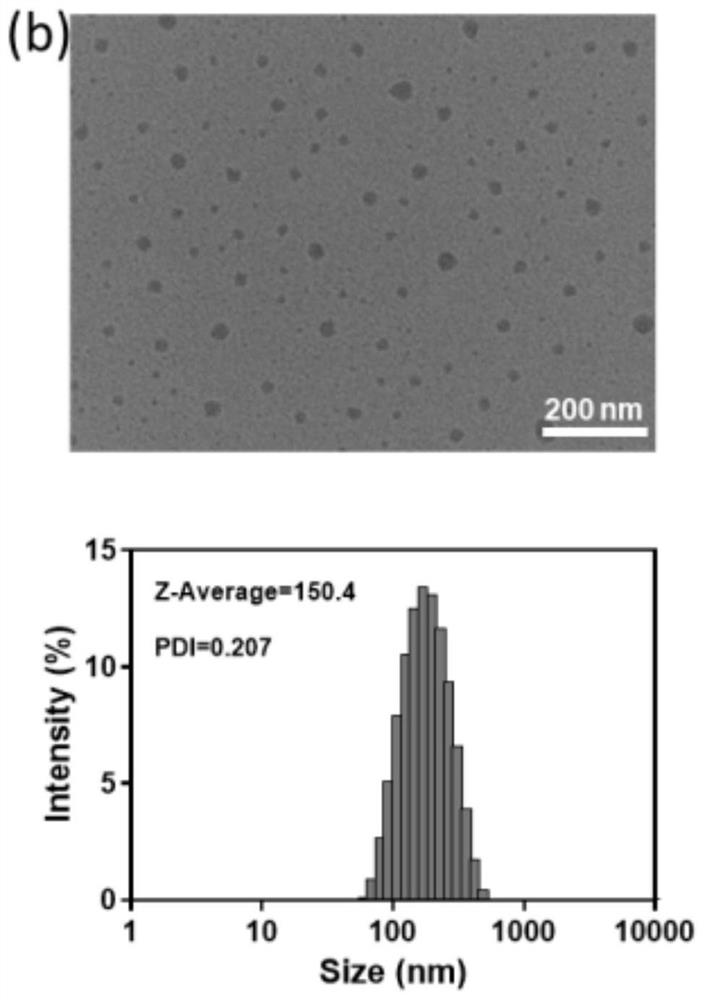
Light-controllable metal ion delivery particle as well as preparation method and application thereof
ActiveCN113893344AThe synthesis method is simpleSynthetic conditions are not harshPowder deliveryPhotodynamic therapyChemical structureEthyl group
The invention discloses a light-controllable metal ion delivery particle as well as a preparation method and application thereof. The light-controllable metal ion delivery particle comprises an organic micromolecular ligand and metal ions combined on the organic micromolecular ligand through coordination, and a chemical structural formula of the organic micromolecular ligand is shown in the specification, wherein R is one of methyl, ethyl and benzyl; and the metal ions are copper ions or cuprous ions. According to the light-controllable metal ion delivery particle, the organic micromolecular ligand can keep the valence state of the metal ions stable, and the metal ions are delivered to a tumor region through a nano self-assembly technology; besides, the organic micromolecular ligand also has the function of a photosensitizer, and the purpose of carrying out photodynamic therapy firstly and then carrying out chemodynamic therapy can be realized, so that accurate light-controlled ion release at the tumor region can be realized, and the toxicity of a whole body is reduced; and the novel drug delivery mode provides a new strategy for collaborative treatment of tumor.
Owner:SHENZHEN UNIV
A kind of ferrous ammonium phosphate, its preparation method and application
ActiveCN111115608BThe preparation method is reasonableStable lattice structureCell electrodesPhosphorus compoundsLithiumFerrous ammonium phosphate
The invention provides a ferrous ammonium phosphate, its preparation method and application, the general formula of ferrous ammonium phosphate is: NH 4 Fe x m (1‑x) PO 4 , wherein x=greater than 0 and less than or equal to 1; M is selected from one or more of V, K, Co, Mn, Zn, Mg, Ni, Al, Ti, Nb, Zr and Cu. The invention also discloses a method for preparing lithium iron phosphate from ferrous ammonium phosphate, which includes the following steps: mixing ferrous salt, phosphorus source and ammonia water, adjusting the pH of the reaction system to 6-7, and reacting to generate ferrous ammonium phosphate; the ferrous The molar ratio of the salt to the phosphorus source is 0.95-1.03:0.95-1.03; the ammonium ferrous phosphate is reduced and calcined at a high temperature under the protection of an inert atmosphere to obtain ferrous pyrophosphate; the ferrous pyrophosphate is mixed with lithium salt and carbon source, Crushing, ball milling; calcining, carbon coating, and synthesis to obtain lithium iron phosphate. The production process of the invention is simple, the production precision is low, and the preparation and doping are easy.
Owner:DALIAN RONGKE ENERGY STORAGE GRP CO LTD
Catalyst for hydrogenation of oxalic ester to ethanol, method of preparing the catalyst, and method of using the same
ActiveUS9029285B2Good dispersionImprove the immunityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCopperBULK ACTIVE INGREDIENT
A catalyst including: a support, the support including a mixture of SiO2 and ZrO2; an active ingredient including copper; a first additive including a metal, an oxide thereof, or a combination thereof; and a second additive including Li, Na, K, or a combination thereof. The metal is Mg, Ca, Ba, Mn, Fe, Co, Zn, Mo, La, or Ce. Based on the total weight of the catalyst, the weight percentages of the different components are as follows: SiO2=50-90 wt. %; ZrO2=0.1-10 wt. %; copper=10-50 wt. %; the first additive=0.1-10 wt. %; and the second additive=0.1-5 wt. %.
Owner:TIANJIN UNIV
Process for recovering and purifying neptunium from waste liquor discharged from 2AW+2DW in Purex flow
ActiveCN103305702BHigh recovery rateValence stableProcess efficiency improvementRadioactive decontaminationOxalate precipitationEngineering
The invention belongs to the technical field of nuclear fuel reprocessing and discloses a process for recovering and purifying neptunium from waste liquor discharged from 2AW+2DW in Purex flow. The process comprises the primary neptunium purification process and the secondary neptunium purification process; the process comprises the following steps: by taking 30 volume percent of TBP-kerosene as an extraction agent, extracting Np, U and Pu to the organic phase, and allowing the fission fragments to enter the aqueous phase; adding a reducing agent and an extraction agent into the organic phase, reversely extracting the Np to the aqueous phase; further extracting uranium and plutonium, and performing oxalate precipitation, filtering and calcining on the obtained aqueous phase product to obtain the purified neptunium oxide solid. The process has the advantages that the neptunium recovery rate is over 98 percent, and the uranium and plutonium removal coefficient in neptunium is high.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Preparation method of D2 structure hexafluoropropylene dimer
PendingCN113929554AEnhanced interactionValence stableHalogenated hydrocarbon preparationFluoroboric acidChemical process
The invention relates to the technical field of chemical processes, in particular to a preparation method of a D2 structure hexafluoropropylene dimer. According to the preparation method of the D2 structure hexafluoropropylene dimer, 5-bromine-6-sulfydryl pyridine and vinyl trifluoroborate are subjected to a sulfydryl-alkene reaction, the prepared auxiliary is added into metal fluoride, the valence state of metal can be stabilized, the catalytic activity can be improved, and high dispersion of active components in a fluorine-containing catalyst is achieved.
Owner:ZHEJIANG JUHUA HANZHENG NEW MATERIAL
A kind of rare earth butyl rubber and its preparation method
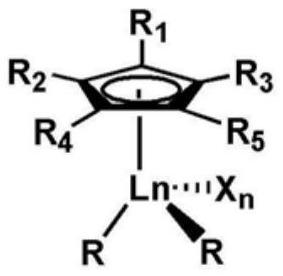
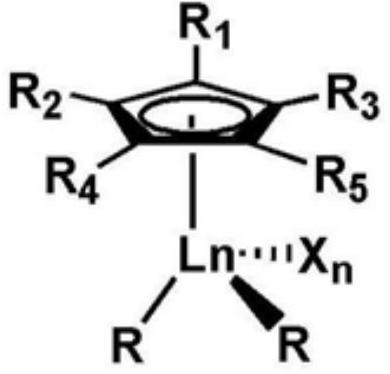
The invention relates to a rare-earth butyl rubber and a preparation method thereof. The rare-earth butyl rubber is a homopolymer prepared by using a rare-earth catalyst to catalyze isobutylene polymerization, or a copolymer prepared by catalyzing isobutylene-comonomer polymerization. The number-average molecular weight is 1-100*10<4>. The mol percent of the comonomer is 1-20%. The comonomer is selected from one or mixture of more of olefins and olefin derivatives, wherein the olefins are selected from alpha-olefins, conjugated olefins and cycloolefins; and the olefin derivatives are selected from alpha-olefin derivatives, conjugated olefin derivatives and cycloolefin derivatives containing nitrogen, oxygen, chlorine and bromine atom functional groups. The rare-earth catalyst comprises A and B, wherein A is rare-earth coordination compounds CpLnR2Xn, Ln is a rare-earth metal, and B is an organic boron reagent. The rare-earth catalyst has the advantages of high activity and stable valence state; the polymerization reaction can be competed at higher temperature; and thus, the method has the advantages of energy saving, high safety, long production cycle, no corrosion, no toxicity and environment friendliness.
Owner:DALIAN UNIV OF TECH
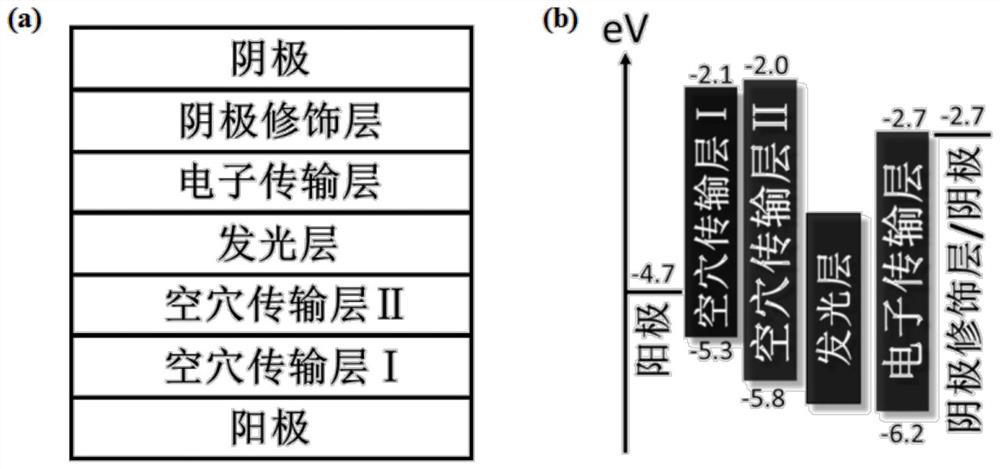
An all-inorganic lead halide perovskite light-emitting diode and its preparation method and active light-emitting layer
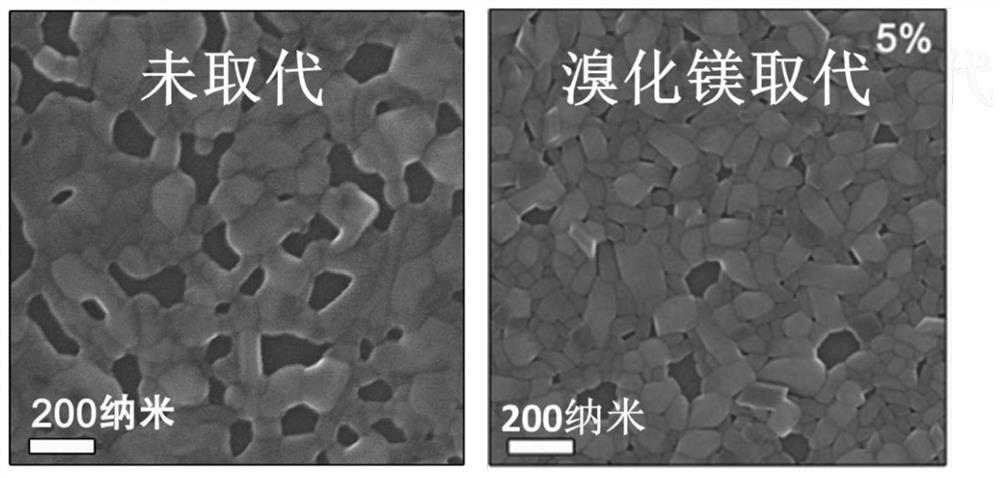
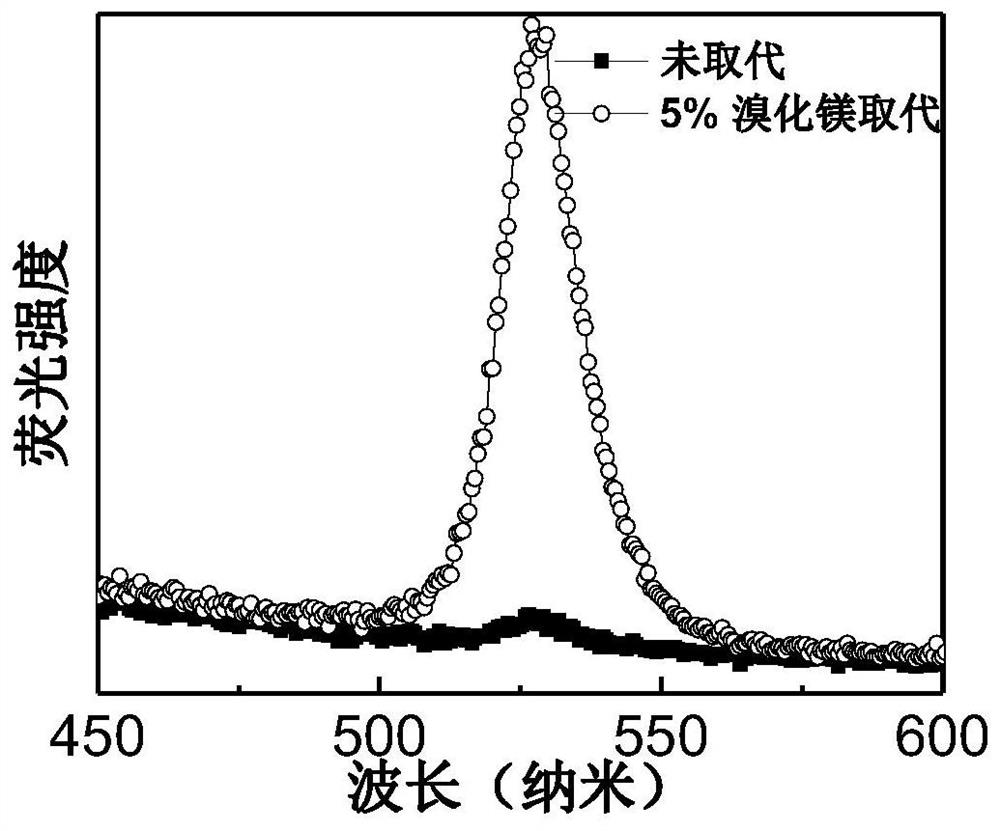
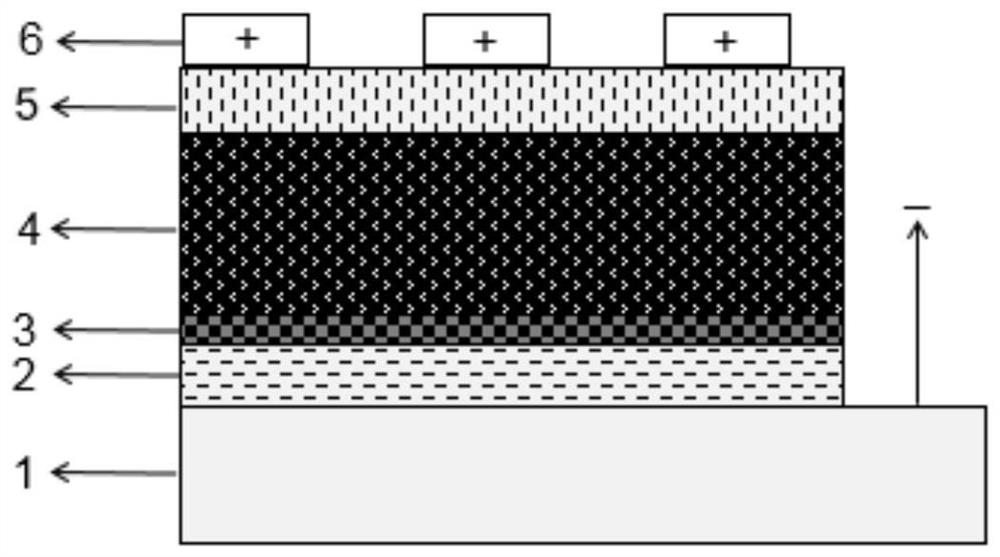
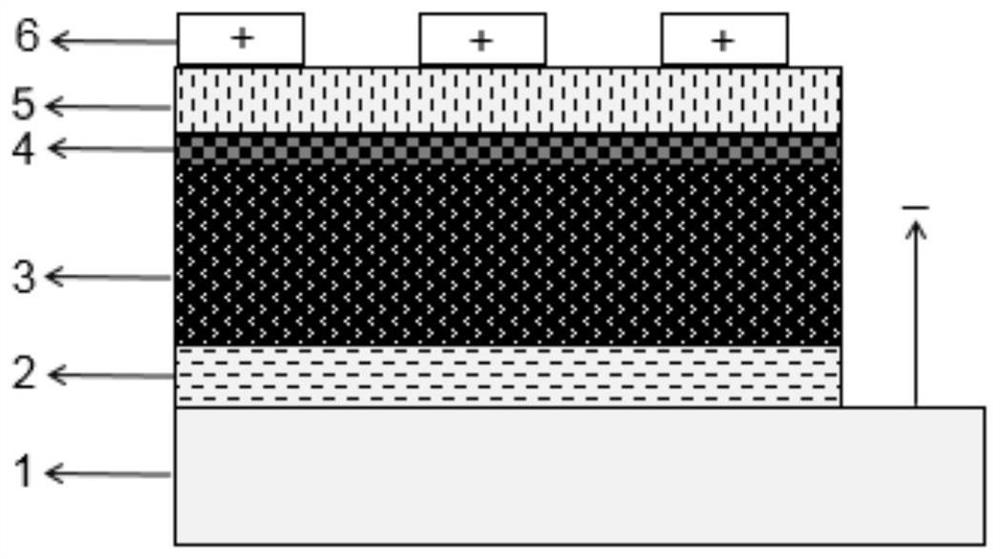
ActiveCN109585694BImprove stabilityLow toxicitySolid-state devicesSemiconductor/solid-state device manufacturingAlkaline earth metalFluorescence
The invention discloses an all-inorganic lead halide perovskite light-emitting diode, which comprises a conductive substrate, a carrier transport layer, an electrode modification layer and an electrode sequentially arranged in a layered structure, wherein the carrier transport layer is arranged in the middle The active luminescent layer is a lead halide perovskite in which part of the lead halide is replaced by an alkaline earth metal halide. In this application, part of the lead halide in the perovskite is replaced by alkaline earth metal. On the one hand, the formation energy of the bromine defect state in the perovskite becomes higher, which means that the bromine defect is more difficult to form, so the defect state of the perovskite is significantly reduced and the fluorescence is enhanced. On the other hand, the morphology of the film is significantly improved, the pores are reduced, and the leakage current is reduced. At the same time, due to the enhanced fluorescence, the brightness is improved, and finally the efficiency of the entire device is improved.
Owner:SUZHOU UNIV
A kind of perovskite photovoltaic cell and preparation method thereof
ActiveCN111710781BLow costAbundant resourcesSolid-state devicesSemiconductor/solid-state device manufacturingPerovskite solar cellPerovskite (structure)
The invention relates to a high-performance perovskite cell and a preparation method thereof. The perovskite solar cell uses Fe prepared by a solution method. 2‑x Mg x O 3 The thin film is used as the interface passivation layer to passivate the adjacent interface of the perovskite photosensitive layer of the perovskite photovoltaic cell, where 0≤x≤0.2, and the thickness is 30-40 nm. The perovskite photovoltaic cell of the present invention uses Fe 2‑x Mg x O 3 For the interface passivation layer, the preparation cost of perovskite cells is greatly reduced, and the interaction between iron and iodine, oxygen and lead is used to improve the crystal quality of perovskite thin films. At the same time, Fe 2‑x Mg x O 3 The thin film can make the carriers move quickly and reduce the charge accumulation at the interface; an appropriate amount of magnesium can also form a strong chemical bond with the surrounding ions, stabilize the structure of the adjacent interface of the perovskite, reduce interface defects, and reduce non-radiative recombination. achieve the purpose of improving device performance.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Anode material of lithium ion cell and preparation method thereof
InactiveCN102280618BHigh rate charge and discharge performance is goodIncrease migration rateCell electrodesElectrical conductorElectrical battery
The invention discloses a preparation method of an anode material of a lithium ion cell, comprising the following steps of: (1) uniformly dispersing a Li source, a Mn source, a Ni source, a doping element and a metal element with superionic conductor attributes to prepare a precursor, wherein the metal element with the superionic conductor attributes is selected from two or two more of Ti, La andZr; (2) drying or igniting the precursor, then carrying out pretreatment to obtain pretreatment powder; (3) pressing the pretreatment powder into a sheet; (4) carrying out heat treatment for 6-24h; and (5) annealing, naturally cooling to room temperature, grinding, and sieving to obtain the anode material. In the invention, on the one hand, a crystal skeleton of the material and a Mn valence state are stabilized by using the doping element, on the other hand, a compound lithium ion superionic conductor improves a migration rate of the lithium ion in the material and improves the multiplying power property and cycling property of an electrode are improved.
Owner:SUZHOU UNIV
Imitated jewel photochromic glass with excellent performance, and preparation method and application thereof
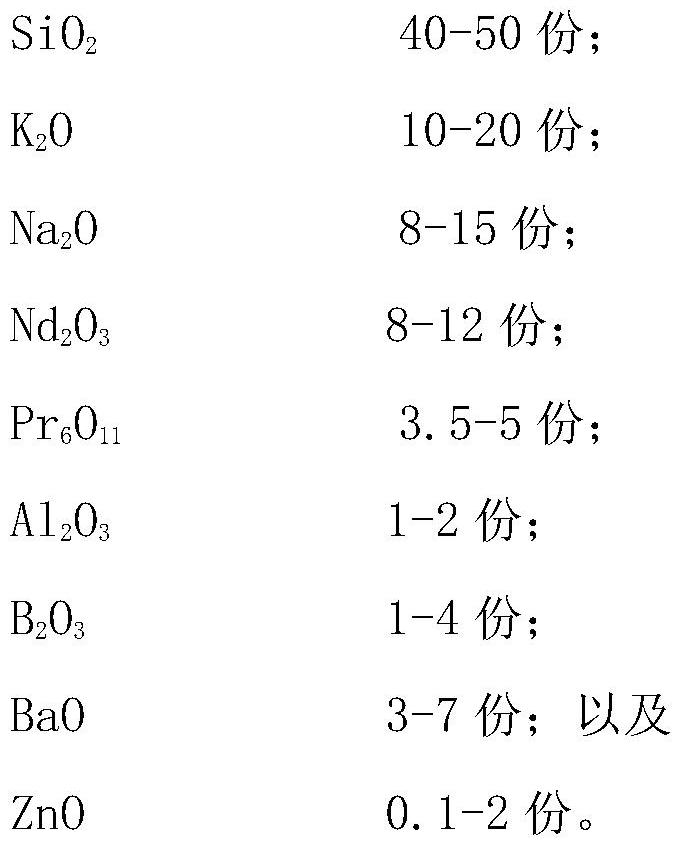
ActiveCN113816603AWith discoloration effectObvious red-green discolorationGlass shaping apparatusGlass productionRare-earth elementMaterials science
The invention relates to an imitated jewel photochromic glass. The imitated jewel photochromic glass comprises, by weight, 30-60 parts of silicon dioxide (SiO2); 5-20 parts of rare earth element oxide; and 0.5-3 parts of aluminum oxide (Al2O3). The imitated jewel photochromic glass has a photochromic effect, the hue difference delta h0 is 20 degrees or above, and the obvious red-green discoloration phenomenon can be observed by naked eyes. The imitated jewel photochromic glass is green or green with yellow color under natural light (D65 light source) due to doping of rare earth elements, is red or red with brown color under an incandescent lamp (A light source), and is in various transition colors between the red color and the green color under a mixed light source. In addition, the invention also provides a preparation method and application of the imitated jewel photochromic glass.
Owner:UNIV OF SCI & TECH BEIJING
A kind of preparation method and application of vanadium trioxide negative electrode material
ActiveCN108557885BHigh specific capacityAvoid contactNegative electrodesSecondary cellsLevel structureLithium-ion battery
The invention belongs to the technical field of lithium ion batteries, and particularly relates to a preparation method and application for a vanadium trioxide negative electrode material. The preparation method for the vanadium trioxide negative electrode material comprises the steps that an ammonium vanadate compound is taken as a precusor substance, a silicon wafer is taken as a carrier, a lithium sheet is taken as a reducing agent, the above substances are placed in a crucible and is calcined in a tube furnace, the above substance is cooled to the indoor temperature naturally, and a V2O3 negative material is obtained. The preparation method is simple and feasible, the production cost is low, and the safety coefficient is high; the prepared V2O3 negative material is of a multi-level structure, and material shape can be controlled. In addition, the prepared V2O3 negative material is used for assembling a half cell, the result shows that the V2O3 negative material is high in specificcapacity, good in rate capability and stable in circular performance. The prepared V2O3 material is taken as the negative material to produce lithium ion batteries and has a wide application prospect.
Owner:JIANGSU UNIV
Laminated magnetic conductive plate raw material composition and production method for making laminated magnetic conductive plate
InactiveCN103131135BGood magnetic performanceAvoid uneven dispersionSynthetic resin layered productsOrganic/organic-metallic materials magnetismEpoxyIron powder
The invention discloses a laminated magnetic conductive plate raw material composition and a production method for manufacturing a modified laminated magnetic conductive plate by utilizing the laminated magnetic conductive plate raw material composition. The laminated magnetic conductive plate raw material composition is a mixer of epoxy resin, iron powder, nano neodymium oxide and a silane coupling agent KH550, wherein the weight ratio of the epoxy resin, the iron powder, the nano neodymium oxide and the silane coupling agent KH550 is 10:2:0.5:0.13, and the proportion of the diluted epoxy resin is 50%. The problem that the iron powder in the laminated magnetic conductive plate on the market is large in proportion, easily precipitates in an epoxy resin solution and is difficult to evenly disperse, and accordingly the magnetic flow rate of the laminated magnetic conductive plate is unstable is solved. The problem that the iron powder is used for a long period of time or is easily oxidized after used in a high-temperature motor, and accordingly the magnetic conductance of the laminated magnetic conductive plate is reduced remarkably is solved.
Owner:焦作市天益科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com