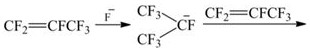
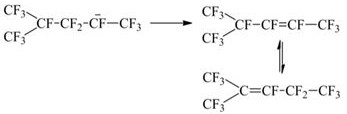
Preparation method of D2 structure hexafluoropropylene dimer
A technology of hexafluoropropylene dimer and hexafluoropropylene, which is applied in the field of chemical technology, can solve the problems of high degree of dissociation and easy aggregation of fluoride anions, and achieve the effect of improving overall performance, reducing the degree of dissociation, and realizing dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
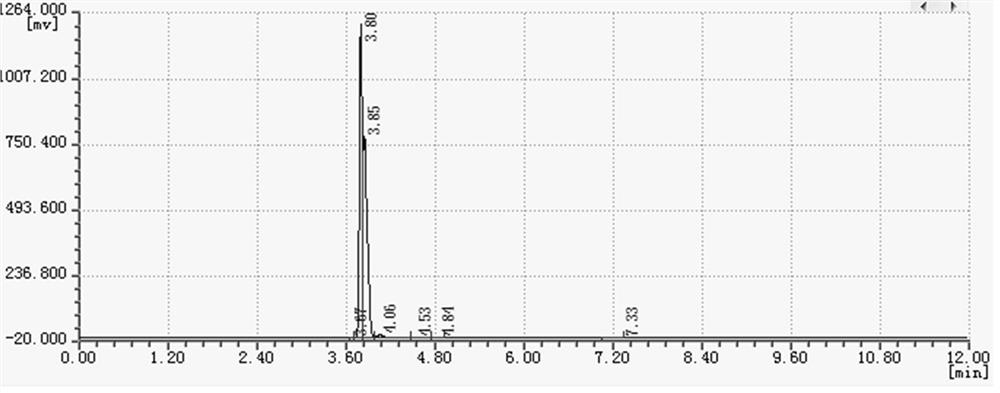
Image
Examples
preparation Embodiment 1
[0043] (1) Add 5g of 3-mercaptopicolinic acid, 3g of vinyl borate pinacol ester, 150g of acetonitrile, add 2g of sodium ethoxide into a closed autoclave, heat up and stir to 50°C, pressure is 0.8MPa, and react for 100min to obtain Auxiliary A;
[0044] (2) Then directly mix the auxiliary agent A with 25 g of potassium fluoride for 10 hours, the molar ratio of the auxiliary agent A to potassium fluoride is 1:1, and distill off the acetonitrile under reduced pressure to obtain the isomerization catalyst 1.
preparation Embodiment 2
[0046] (1) Add 12g of 3-mercaptopicolinic acid, 6g of vinylboronic acid pinacol ester, 220g of acetonitrile into a closed high-pressure reactor, add 6g of sodium ethoxide, heat up and stir to 65°C, pressure is 2.0MPa, and react for 150min to obtain Auxiliary A;
[0047] (2) Then directly mix Auxiliary A with 36 g of potassium fluoride for 20 hours, the molar ratio of A and potassium fluoride is 1:1.5, and distill off acetonitrile under reduced pressure to obtain isomerization catalyst 2.
Embodiment 1
[0049] (1) Oligomerization reaction
[0050] Cesium fluoride was calcined at 400°C for 10 hours, ground into powder and added to the autoclave together with anhydrous acetonitrile, stirred and mixed evenly, then bubbled with argon for 5 minutes, then vacuumed, and argon was circulated for 3 times. Fill 20g of hexafluoropropylene under the conditions, control the temperature at 30°C, pressure at 0.8MPa, stir the reaction for 2 hours, and the stirring speed at 50r / min, cool down to 10°C after the reaction is completed, and then discharge the residual hexafluoropropylene gas, Liquid obtains lower floor liquid; Join in the autoclave;
[0051] (2) Isomerization reaction
[0052] Add 0.5g of isomerization catalyst 1 into the autoclave, raise the temperature to 100°C under the protection of nitrogen, and react for 60 minutes. After the reaction is completed, cool to room temperature and separate the catalyst to obtain the highly reactive D2 structure hexafluoro Propylene dimer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com