Anode material of lithium ion cell and preparation method thereof
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as poor rate charge and discharge performance, deterioration of electrochemical performance, decline in material specific capacity and rate performance, and achieve long-term improvement. Good cycle performance and high temperature performance, good electrochemical cycle stability, good high rate charge and discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Take LiAc·2H 2 O, Ni(Ac) 2 4H 2 O, MnAc 2 4H 2 O is the basic raw material, Ti(OC 4 h 9 ) 4 Ti in is a doping element, Ti and La(NO 3 ) 3 ·6H 2 La in O is a metal element with superionic conductor properties, and the corresponding aqueous solutions are respectively configured, and the molar ratio of each metal element is Li:Mn:Ni:La:Ti=20:27:9:1:2;
[0038] Then configure a mixed solution of citric acid and ethylene glycol with a molar ratio of 1:4, and heat it to 90°C under constant stirring. When the solution is transparent and clear, add the above aqueous solution drop by drop. The molar ratio is 1:1, and the solution is incubated at 90°C for 1 to 2 hours until the gelation process is completed;
[0039] After the sample is completely dried, it is ignited, and the sample is ground and placed in a muffle furnace for pretreatment at 500 °C;
[0040] Then use 20atm / cm 2 Tablets were pressed under the pressure of 750°C for 12 hours; after natural cooling, the...
Embodiment 2
[0042] With Li 2 CO 3 , Ni(NO 3 ) 2 ·6H 2 O, MnAc 2 4H 2 O is the basic raw material, Ti(OC 4 h 9 ) 4 Ti in is a doping element, Ti and La(NO 3 ) 3 ·6H 2 La in O is a metal element with superionic conductor properties, respectively configure corresponding aqueous solutions, the molar ratio of each metal element Li:Mn:Ni:La:Ti=20:27:9:1:2; put the above raw materials Ball milling in ethanol, acetone or other organic medium for 10h;
[0043] The ball-milled and dried samples were pretreated at 350°C for 8 hours, and then 20 atm / cm 2 After pressing into tablets, keep it at 800°C for 12 hours, after natural cooling, take out the sample and grind it again, then keep it at 750°C for 12 hours, then anneal at 600°C for 12 hours, cool naturally, grind and sieve, get Ti doping and Li 0.5 La 0.5 TiO 3 Composite LiMn as Li-ion superionic conductor 1.5 Ni 0.5 o 4 Cathode material.
Embodiment 3
[0045] Take LiOH·H 2 O, Mn(NO 3 ) 2 4H 2 O, Ni(NO 3 ) 2 ·6H 2 O is the basic raw material, TiCl 4 Ti in is a doping element, Ti and La(NO 3 ) 3 ·6H 2 La in O is a metal element with superionic conductor properties, a precursor mixed in deionized water according to the molar ratio of each metal element Li:Mn:Ni:La:Ti=20:27:9:1:2 slurry;
[0046] Evaporated and dried at 200°C, then pretreated with air flow at 500°C to fully dry the sample, and then use 20atm / cm 2 The pressure tablet was pressed at 800°C for 12 hours. After natural cooling, the sample was taken out and re-grinded. Then, it was kept at 700°C for 12 hours, and then annealed at 600°C for 6 hours. After natural cooling, it was ground and 400 mesh Sieve to get Ti-doped and Li 0.5 La 0.5 TiO 3 Composite LiMn as Li-ion superionic conductor 1.5 Ni 0.5 o 4 Cathode material.
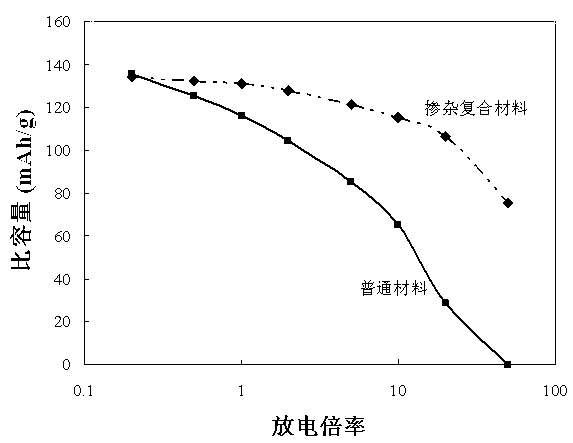
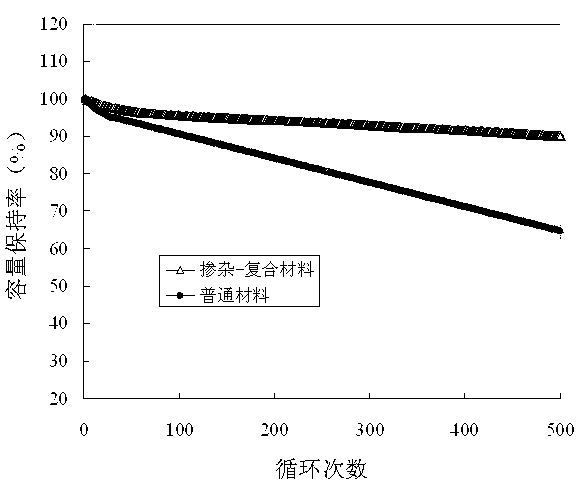
[0047] The Ti doping and Li 0.5 La 0.5 TiO 3 Composite LiMn as Li-ion superionic conductor 1.5 Ni 0.5 o 4 Electrochemical p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com