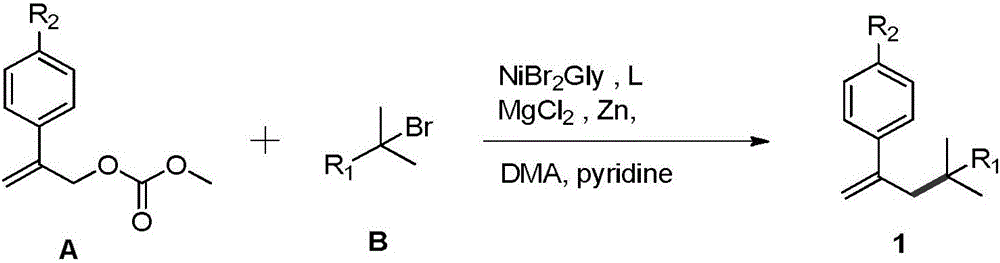
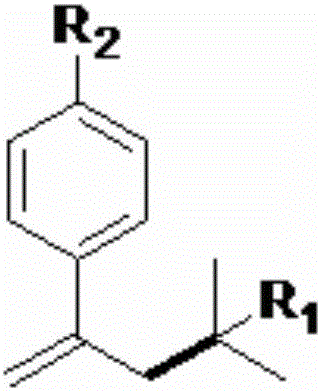
Styrenic quaternary carbon compound and preparation method thereof
A technology of styrene and carbon compounds, which is applied in the preparation of organic compounds, the production of hydrocarbons from oxygen-containing organic compounds, and the production of hydrocarbons from halogen-containing organic compounds, etc. problems such as poor adaptability to animals, to achieve the effects of low price, good adaptability and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
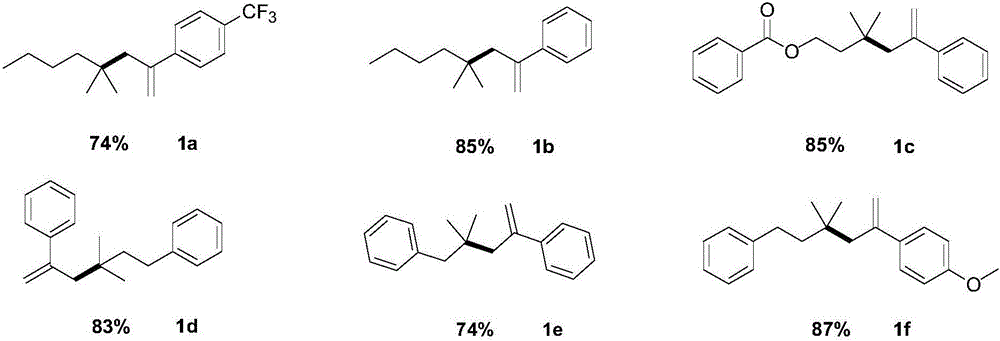
[0019] Example 1: 1-(4,4-Dimethyloctyl-1-en-2-yl)-4-(trifluoromethyl)benzene
[0020] 1-(4,4-dimethyloct-1-en-2-yl)-4-(trifluoromethyl)benzene
[0021]
[0022] Add Zn powder (0.9mmol, 300%), ligand 2,6-bis-(4-isopropyl-4,5-dihydrooxazol-2-yl) in a dry Schlenk reaction flask equipped with magnetons Pyridine (iPr-box, 0.045mmol, 15%), MgCl 2 (0.3 mmol, 100%). Cover the rubber stopper and repeatedly evacuate and flush nitrogen three times, then add nickel bromide ethylene glycol dimethyl ether (NiBr 2 .Glyme, 0.03mmol, 10%), then inject solvent N, N-dimethylacetamide (DMA, 1ml), carbonic acid 2-(4-substituted phenyl) allyl methyl ester (methyl(1-(4 -(trifluoromethyl)phenyl)vinyl)carbonate, liquid, 0.6mmol, 200%), tertiary halogenated hydrocarbon (2-bromo-2-methylhexane, liquid, 0.3mmol, 100%), pyridine (0.09mmol, 30%) . The reaction was stirred at 20-25 °C for 8-12 h under the protection of nitrogen. After the reaction, the target product (63.0mg, 0.222mmol, 74% yield) ...
example 2
[0024] Example 2: (4,4-Dimethyloctyl-1-en-2-yl)benzene
[0025] (4,4-dimethyloct-1-en-2-yl)benzene
[0026]
[0027] Add Zn powder (0.9mmol, 300%), ligand 2,6-bis-(4-isopropyl-4,5-dihydrooxazol-2-yl) into a Schlenk reaction bottle equipped with magneton drying Pyridine (iPr-box, 0.045mmol, 15%), MgCl 2 (0.3 mmol, 100%). Cover the rubber stopper and repeatedly evacuate and flush nitrogen three times, then add nickel bromide ethylene glycol dimethyl ether (NiBr 2 .Glyme, 0.03mmol, 10%), inject solvent DMA (1ml) then, carbonic acid 2-(4-substituted phenyl) allyl methyl ester (methyl(1-phenylvinyl)carbonate, liquid, 0.6mmol, 200%) , tertiary haloalkane (2-bromo-2-methylhexane, liquid, 0.3mmol, 100%), pyridine (0.09mmol, 30%). The reaction was stirred at 20-25 °C for 8-12 h under the protection of nitrogen. After the reaction, the target product (55.1 mg, 0.255 mmol, 85% yield) was directly obtained by column chromatography.
[0028] The product is a colorless oily liquid....
example 3
[0029] Example 3: 3,3-Dimethyl-5-phenylhexyl-5-en-1-ylbenzoate
[0030] 3,3-dimethyl-5-phenylhex-5-en-1-yl benzoate
[0031]
[0032] Add Zn powder (0.9mmol, 300%), ligand 2,6-bis-(4-isopropyl-4,5-dihydrooxazol-2-yl) into a Schlenk reaction bottle equipped with magneton drying Pyridine (iPr-box, 0.045mmol, 15%), MgCl 2 (0.3 mmol, 100%). Cover the rubber stopper and repeatedly evacuate and flush nitrogen three times, then add nickel bromide ethylene glycol dimethyl ether (NiBr 2 .Glyme, 0.03mmol, 10%), then inject solvent DMA (1ml), carbonic acid 2-(4-substituted phenyl) allyl methyl ester (methyl(1-phenylvinyl)carbonate, 0.6mmol, liquid, 200%) , Tertiary halogenated alkanes (3-bromo-3-methylbutyl benzoate, liquid, 100%), pyridine (0.09mmol, 30%). The reaction was stirred at 20-25 °C for 8-12 h under the protection of nitrogen. After the reaction, the target product (78.5mg, 0.255mmol, 85% yield) was directly separated by column chromatography.
[0033] The product is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com