Light-controllable metal ion delivery particle as well as preparation method and application thereof
A metal ion and particle delivery technology, applied in the field of biomedical materials, can solve problems such as poor stability of cuprous ions, achieve the effects of less harsh synthesis conditions, reduced systemic toxicity, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of nanoparticles of different valence copper ion delivery systems
[0052] Dissolve 1mg of ligand molecules in 1mL of dichloromethane, first add 100μL of cuprous ions (20mM), mix with ultrasonic, then add different amounts of DSPE-PEG2000 (10mg), after ultrasonic for 30s, add the above liquid to 5mL of supernatant Pure water, then sonicate for 5 minutes, remove dichloromethane by rotary evaporation, pass through a 220 μm PES membrane, transfer to a 30kD ultrafiltration tube, centrifuge at 3500 rpm, 15 minutes, 4°C. The obtained LC1 was washed twice with water and stored at 4°C in the dark for future use.
[0053] Replace the cuprous ions with the same amount of copper ions, and the subsequent operation steps remain unchanged to obtain LC2, which is stored at 4°C in the dark for future use.
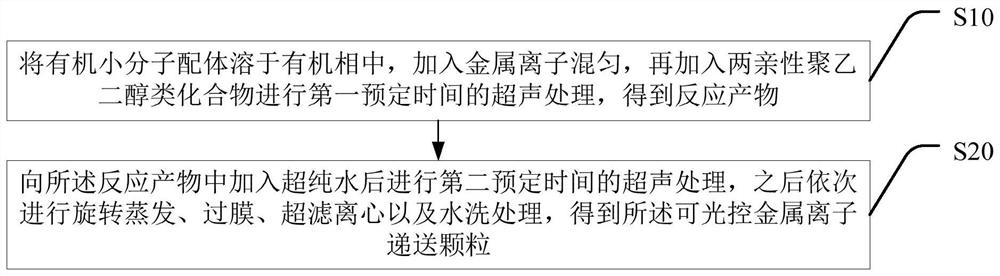
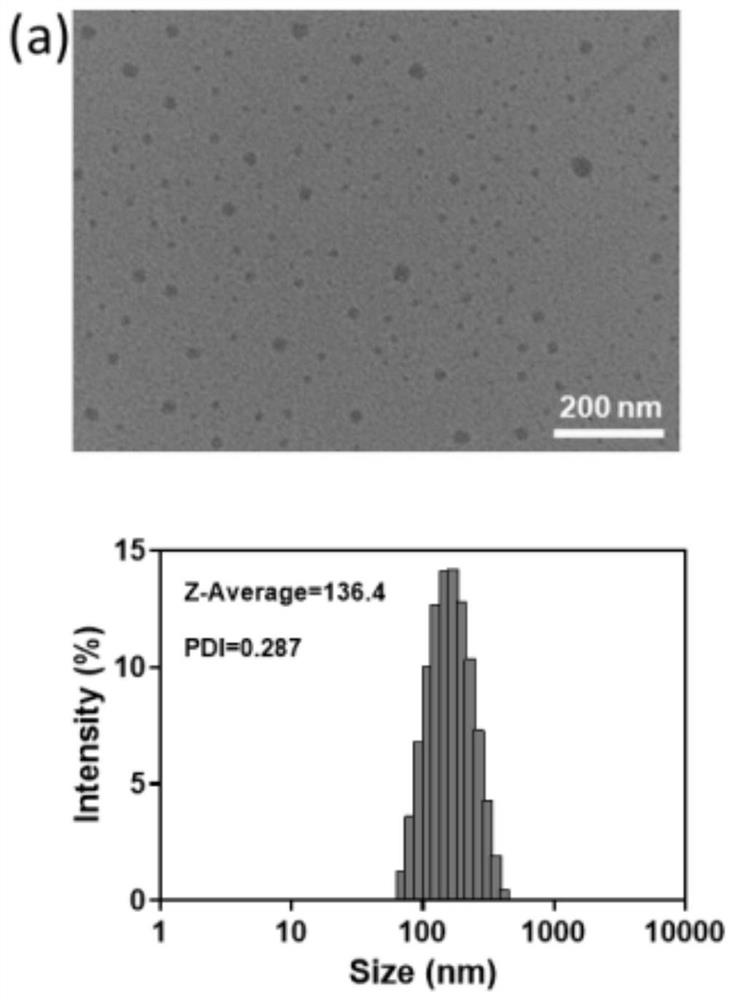
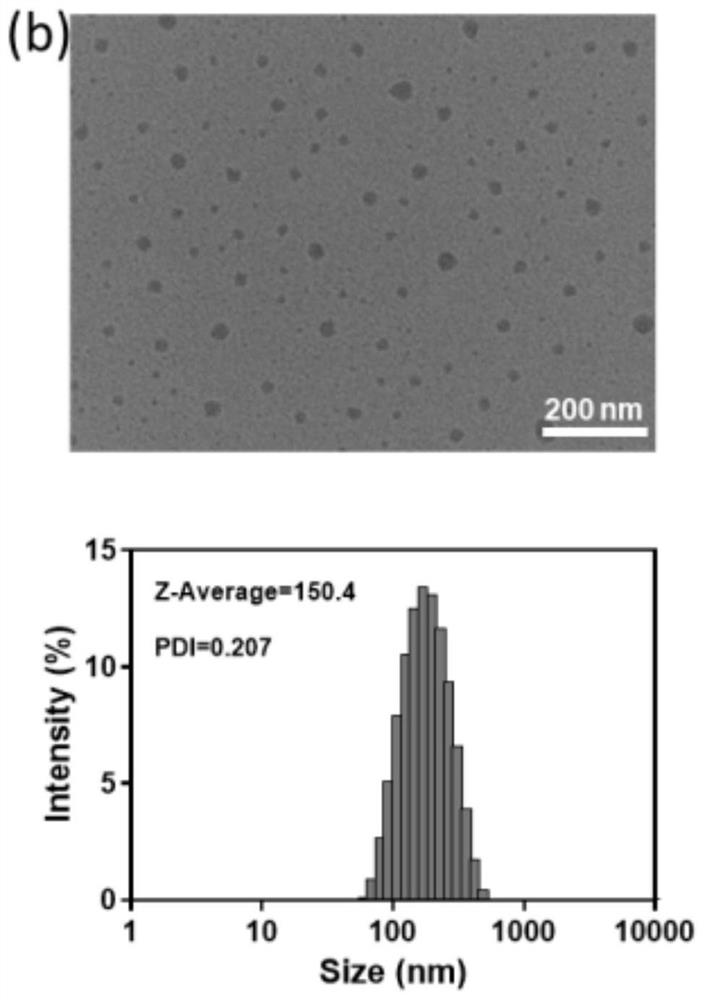
[0054] Figure 2a It is the transmission electron microscope image and the corresponding hydrated particle size image of the prepared nanoparticles (LC1) co...
Embodiment 2
[0057] Example 2: Photodynamic effect evaluation of different valence copper ion delivery systems
[0058] Use a 660nm laser to irradiate the nanoparticle solution after adding DPBF, and the irradiation power is 0.2W / cm 2 , and the duration of each exposure is 10 seconds.
[0059] Figure 5 It shows the DPBF absorption changes of LC1 and LC2 at the same concentration (20 μM) under the same light power. DPBF can be used to detect singlet oxygen ( 1 o 2 ), the faster the absorption value at 415nm decreases, the more singlet oxygen is produced. Figure 5 The results show that LC1 can produce a stronger photodynamic effect than LC2.
Embodiment 3
[0060] Example 3: Evaluation of chemical kinetic effects of copper ion delivery systems in different valence states
[0061] The effects of hydroxyl radicals produced by different valence state copper ion delivery systems under different concentrations and different light powers were compared respectively. The specific conditions are as follows: add 10 μL of 30% hydrogen peroxide solution to 6 mM terephthalic acid (TA) solution, then add LC1 or LC2, each 5 μM, under different light power irradiation, monitor the concentration of TA by fluorescence spectrophotometer Changes in fluorescence intensity at 315 nm. And different concentrations of LC1 at 660nm laser 0.2W / cm 2 The change of the fluorescence intensity of TA at 315nm when the power is irradiated; the change of the fluorescence intensity of TA at 315nm under the same concentration of laser irradiation with different laser powers.
[0062] Figure 6a Indicates the comparison of the efficiencies of hydroxyl radicals pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com