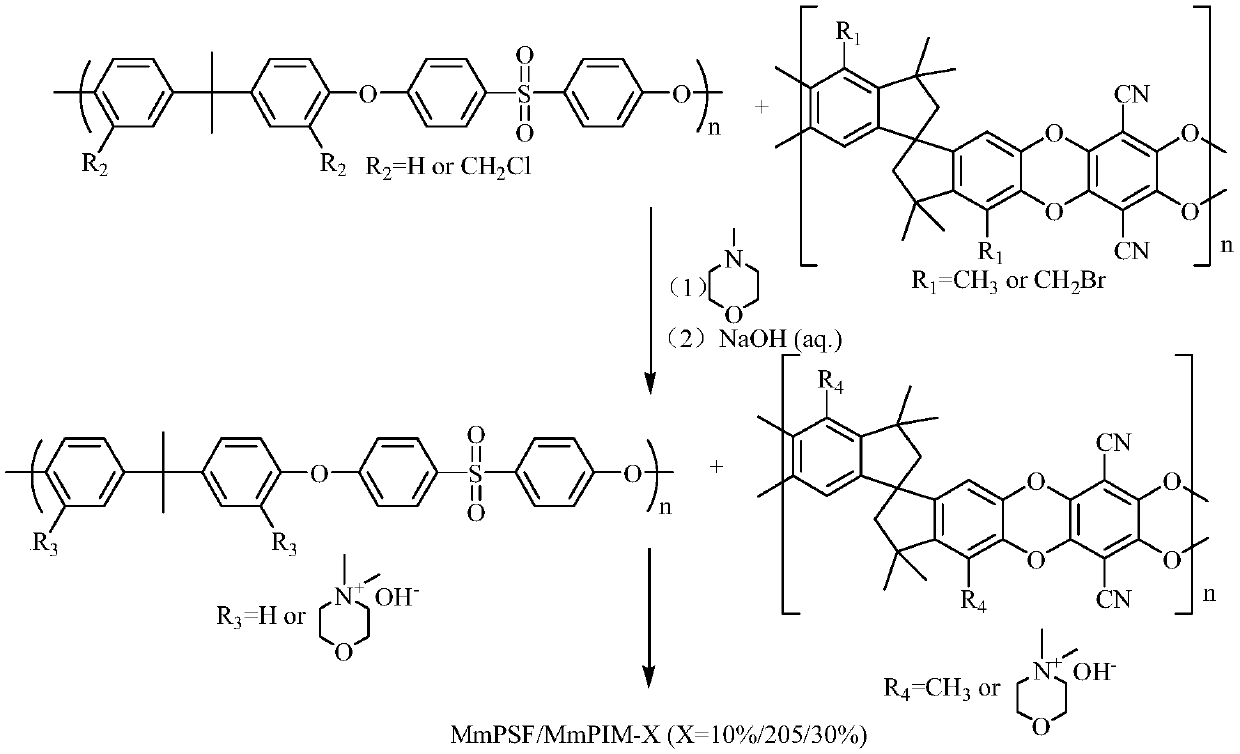
A blended anion exchange membrane and a preparation method thereof
An anion exchange membrane, blending technology, used in final product manufacturing, sustainable manufacturing/processing, fuel cells, etc., can solve problems such as unsystematic water absorption, and achieve good chemical stability and high electrical conductivity. , the effect of high dimensional stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
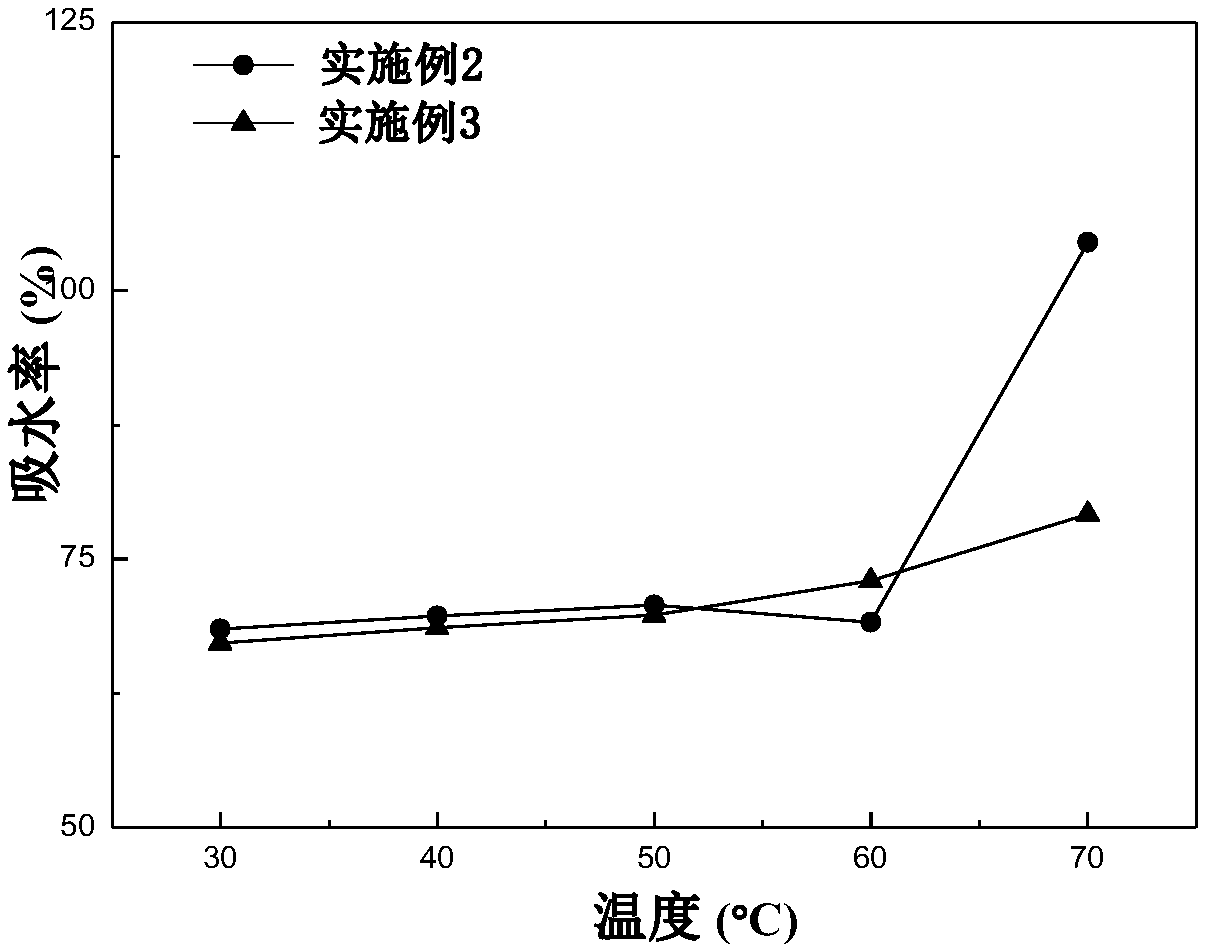
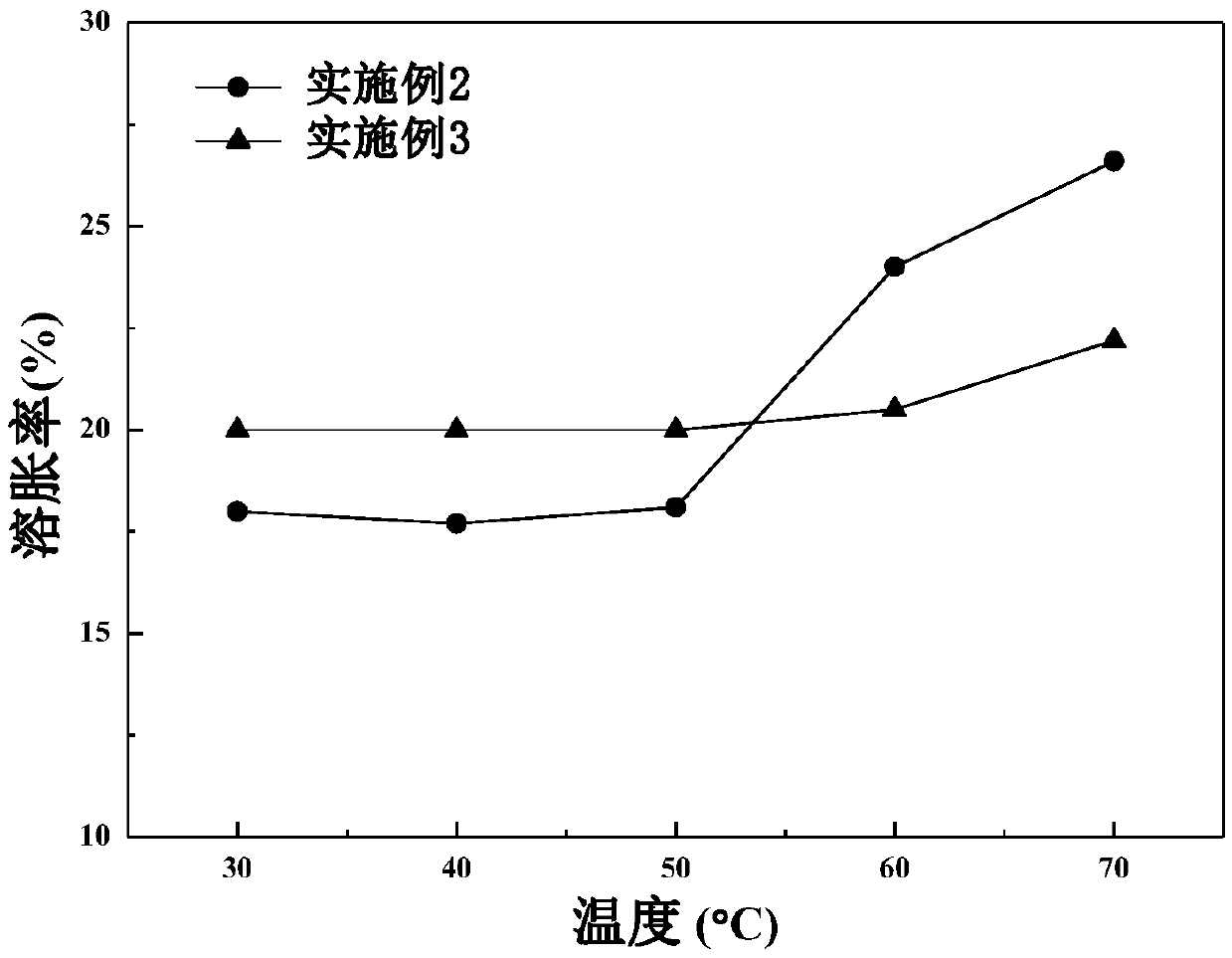
Examples
Embodiment 1
[0046] 8.4 g of 1,2-dihydroxy-3-methylbenzene were dissolved in 18 ml of aqueous hydrobromic acid and 16.5 ml of acetic acid solution to obtain a clear slightly yellowish solution. 10.5ml of acetone was added dropwise to the mixture, and the obtained transparent solution was heated to 110°C and magnetically stirred and refluxed for 36 hours. After the reaction was completed, the mixture was slowly poured into 500ml of deionized water, washed and filtered, and vacuum-dried at 30°C for 24 hours to obtain a black color. solid. Put the black solid in 200ml of acetic acid, stir and wash it for 3 hours, filter it with suction, and dry it under vacuum at 30°C for 24 hours to obtain a white solid which is the product 5,5',6,6'-tetrahydroxy-3,3,3',3', 7,7'-Hexamethyl-helical bisindane (THSBI).
[0047] Weigh 5.425g of THSBI and dissolve it in 67.5ml of anhydrous N,N-dimethylformamide (DMF) to obtain a yellow transparent solution, and add 18g of tert-butyldimethylsilyl chloride. The m...
Embodiment 2
[0055] 16.8 g of 1,2-dihydroxy-3-methylbenzene was dissolved in 36 ml of aqueous hydrobromic acid and 33 ml of acetic acid solution to obtain a clear slightly yellowish solution. 21ml of acetone was added dropwise to the mixed solution, and the obtained transparent solution was heated to 120°C and magnetically stirred and refluxed for 24 hours. After the reaction was completed, the mixed solution was slowly poured into 500ml of deionized water while hot, washed three times, suction filtered, and vacuum-dried at 50°C for 24 hours to obtain black solid. Put the black solid in 200ml of acetic acid, stir and wash it for 3 hours, filter it with suction, and dry it under vacuum at 50°C for 24 hours to obtain a white solid which is the product 5,5',6,6'-tetrahydroxy-3,3,3',3', 7,7'-Hexamethyl-helical bisindane (THSBI).
[0056] Weigh 10.85g of THSBI and dissolve it in 135ml of anhydrous N,N-dimethylformamide (DMF) to obtain a yellow transparent solution, and add 36g of tert-butyldim...
Embodiment 3
[0065] 13.44 g of 1,2-dihydroxy-3-methylbenzene were dissolved in 28.8 ml of aqueous hydrobromic acid and 26.4 ml of acetic acid solution to obtain a clear slightly yellowish solution. 16.8ml of acetone was added dropwise to the mixture, and the obtained transparent solution was heated to 130°C and magnetically stirred and refluxed for 15 hours. After the reaction was completed, the mixture was slowly poured into 500ml of deionized water while it was still hot, washed and filtered, and vacuum-dried at 80°C for 36 hours to obtain a black color. solid. Put the black solid in 200ml of acetic acid, stir and wash it for 3 hours, filter it with suction, and dry it under vacuum at 80°C for 36 hours to obtain a white solid which is the product 5,5',6,6'-tetrahydroxy-3,3,3',3', 7,7'-Hexamethyl-helical bisindane (THSBI).
[0066] Weigh 8.68g of THSBI and dissolve it in 108ml of anhydrous N,N-dimethylformamide (DMF) to obtain a yellow transparent solution, and add 28.8g of tert-butyldim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com