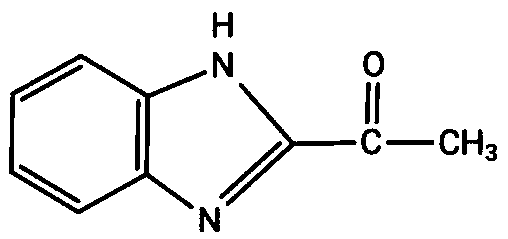
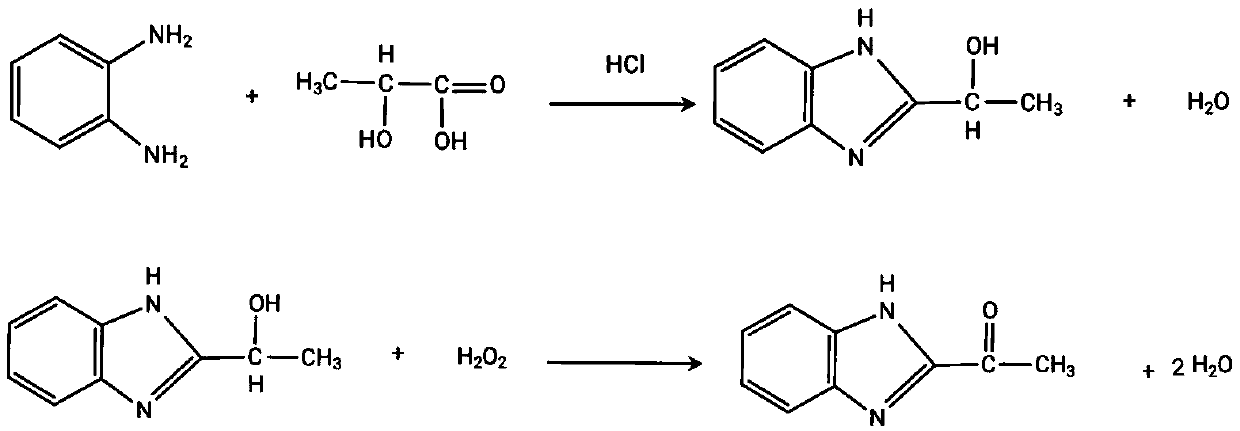
Preparation method for benzimidazolone
A technology of benzimidazolone and hydroxyethyl benzimidazole is applied in the field of preparation of benzimidazolone, can solve problems such as high cost, waste of resources, increase in cost, etc., achieves simplified synthesis process and operation steps, and saves time and cost , the effect of reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Pass 50g of o-phenylenediamine and a certain amount of HCl solution into the heterogeneous catalytic oxidation reaction device with copper, zinc and zirconium as composite catalysts and alumina as the carrier in proportion, slowly add 42g of lactic acid dropwise, and the reaction ends Afterwards, the pH value was adjusted to obtain 2-α-hydroxyethylbenzimidazole, which was filtered and dried to obtain 67.3 g of the product, with a content of 98.5% and a yield of 94.7%.
[0024] 2. Put 50g of 2-α-hydroxyethylbenzimidazole into acetone and hydrochloric acid and a heterogeneous loading catalytic oxidation reaction device to obtain 49.4g of product 2-acetylbenzimidazole after the reaction, with a content of 98.4% and a yield of 98.5 %.
Embodiment 2
[0026] 1. Put 100g of o-phenylenediamine and a certain amount of HCl solution in a heterogeneous catalytic oxidation reaction device loaded with copper-zirconium composite catalyst and pure silica as a carrier, slowly add 84g of lactic acid dropwise, and adjust the pH value after the reaction 2-α-Hydroxyethylbenzimidazole was filtered and dried to obtain 145.7 g of the product, with a content of 98.1% and a yield of 95.3%.
[0027] 2. Put 140g of 2-α-hydroxyethylbenzimidazole into acetone, hydrochloric acid and heterogeneous loading catalytic oxidation reaction device, after the reaction, 137.2g of product 2-acetylbenzimidazole was obtained, with a content of 98.9%, and a yield of 98.1 %.
Embodiment 3
[0029] 1. Put 20g of o-phenylenediamine and a certain amount of HCl solution in a heterogeneous catalytic oxidation reaction device loaded with copper and zirconium as a composite catalyst and alumina as a carrier, slowly add 16.8g of lactic acid dropwise, and adjust the pH after the reaction 2-α-Hydroxyethylbenzimidazole was obtained, and 28.0 g of the product was obtained after filtration and drying, with a content of 99.0% and a yield of 92.4%.
[0030] 2. Put 28g of 2-α-hydroxyethylbenzimidazole into acetone and hydrochloric acid and a heterogeneous loading catalytic oxidation reaction device, after the reaction, 27.0g of the product 2-acetylbenzimidazole was obtained, with a content of 98.7% and a yield of 96.5 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com