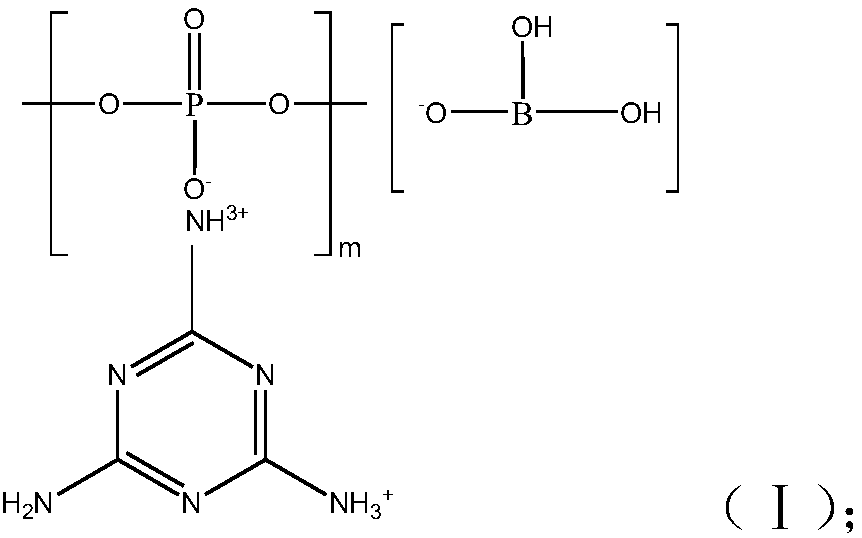
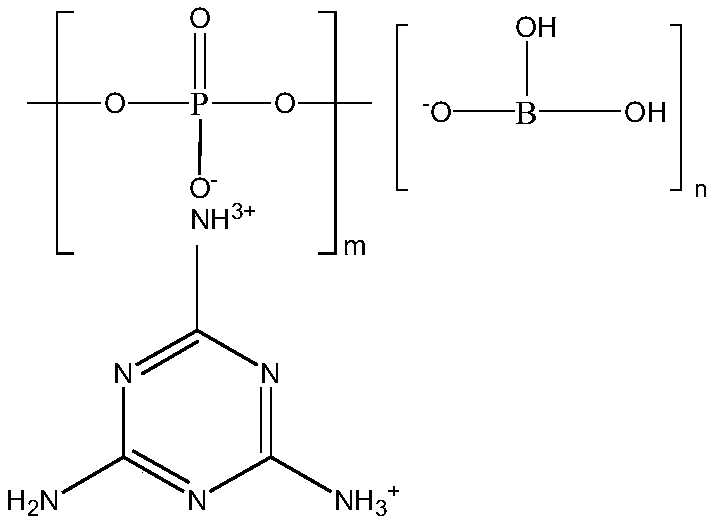
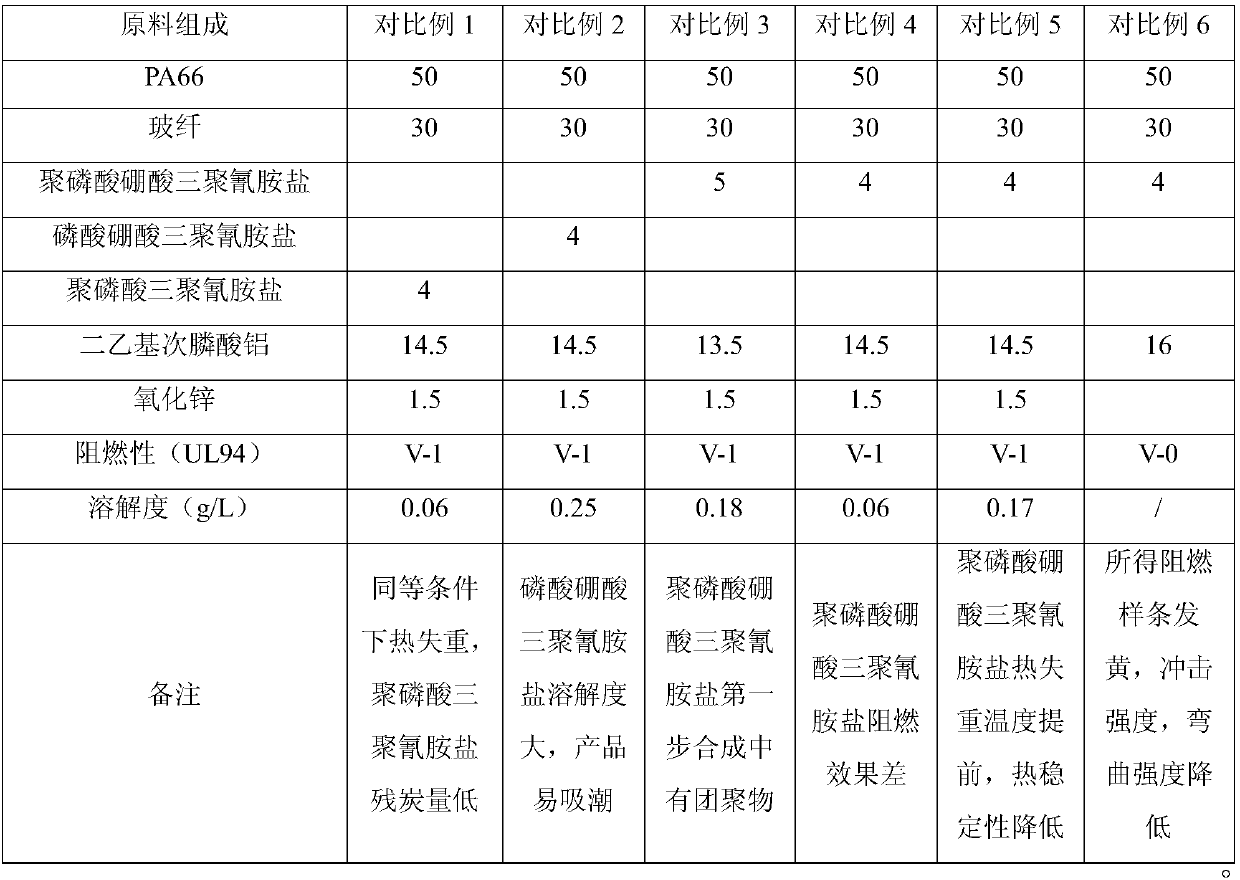
Halogen-free flame-retardant glass fiber reinforced nylon
A nylon and glass fiber technology, applied in the field of glass fiber reinforced nylon, can solve the problems of low flame retardant efficiency, easy generation of phosphine, and decline in mechanical properties of finished products, so as to improve flame retardant performance, excellent flame retardant efficiency, The effect of improving the flame retardant efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] a. Add 500mL of deionized water and 186g of melamine to a 1000mL four-neck flask equipped with a stirrer, thermometer and reflux condensing device, heat up and stir to 80°C, then slowly add 199g of 85% phosphoric acid dropwise, within about one hour The dropwise addition ends. Then keep stirring at 95°C for 2 hours. The obtained slurry was filtered, washed several times with deionized water, and dried at 120° C. to obtain 323.7 g of an intermediate.
[0060] b. Add 1000mL of deionized water and 27.1g of boric acid to a 2000mL four-necked flask equipped with a stirrer, a thermometer and a reflux condensing device, heat up to 80°C to completely dissolve the boric acid, add the 323.7g intermediate obtained in step a, and keep The temperature was 80°C, and the reaction was stirred for 3 hours. The suspension was cooled, filtered and washed several times with deionized water, then dried at 120°C to yield 316.4 g of intermediate product.
[0061] c. Heat-treat 316.4 g of t...
Embodiment 2
[0087] a. Add 500mL of deionized water and 186g of melamine to a 1000mL four-neck flask equipped with a stirrer, thermometer and reflux condensing device, heat up and stir to 60°C, slowly add 154g of 85wt% phosphoric acid dropwise, within about one hour Plus end. Stirring at 80° C. for 1 hour to complete the reaction, the obtained slurry was filtered, washed with deionized water several times, and then dried at 120° C. to obtain 289.9 g of an intermediate.
[0088] b. Add 800mL of deionized water to a 2000mL four-neck flask equipped with a stirrer, thermometer and reflux condensing device, add 31.7g of boric acid, heat up and stir to completely dissolve the boric acid, continue to heat up to 90°C, and add the obtained in step a 289.9g of the intermediate was then reacted with stirring at 100°C for 3.5h. The suspension was cooled, filtered and washed several times with deionized water, then dried at 120°C to yield 289.5 g of intermediate product.
[0089] c. Heat-treat 289.5 ...
Embodiment 3
[0093] a. Add 500mL of deionized water and 186g of melamine to a 1000mL four-necked flask equipped with a stirrer, a thermometer and a reflux condensing device, heat up and stir to 100°C, slowly add 199g of 85wt% phosphoric acid dropwise, within about one hour Plus end. Stirring at 90° C. for 2 hours to complete the reaction, the obtained slurry was filtered, washed with deionized water several times, and then dried at 120° C. to obtain 332.1 g of an intermediate.
[0094] b. Add 1000mL of deionized water and 31.1g of boric acid to a 2000mL four-necked flask equipped with a stirrer, thermometer and reflux condensing device, heat up and stir to completely dissolve the boric acid, continue to heat up to 100°C, and add 332.1g of boric acid obtained in step a g intermediate, keep the temperature at 92°C, and stir the reaction for 3h. The suspension was cooled, filtered, washed several times with deionized water, and dried at 140°C to yield 313.9 g of intermediate product.
[009...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com