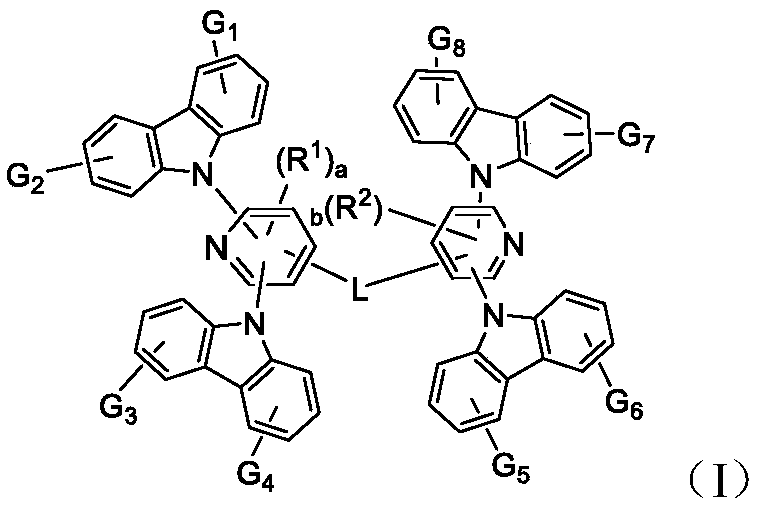
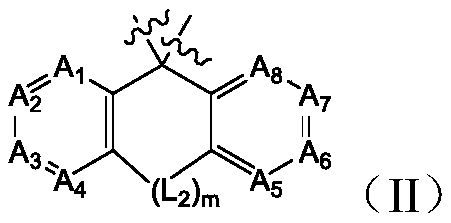
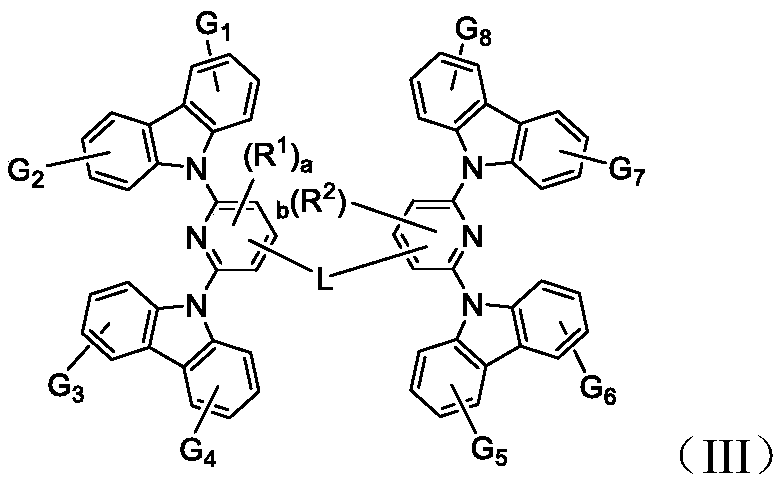
Bis-carbazole pyridine compound and applications thereof
A biscarbazolpyridine and compound technology, applied in the field of organic electroluminescent materials, can solve the problems of low triplet energy level, insufficient glass transition temperature, poor thermal stability, etc., and achieve good luminescence performance, long device life, and reaction The effect of simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114]
[0115] 2,6-Difluoro-4-bromopyridine (1.93g, 10mmol) and carbazole (3.7g, 22mmol) were added to a round bottom flask, and then potassium tert-butoxide (2.46g, 22mmol), N-methyl Pyrrolidone 20mL, after evacuating nitrogen, heat up to 110 degrees, react for 24 hours, cool to room temperature, add 100mL water, dichloromethane separate liquid extraction three times, combine the organic phase, concentrate the organic phase after drying, mix the sample with silica gel and pass through the column . 2.3 g of solid intermediate (M-1) were obtained.
[0116]
[0117] 9,9-dihydrofluorene (1mmol), intermediate (M-1) (2mmol), potassium tert-butoxide (2mmol), palladium acetate (5mol%), tricyclohexylphosphine (10mol%), anhydrous toluene 50mL, reacted at 80°C for 24 hours, cooled to room temperature, separated and extracted three times with dichloromethane, combined the organic phase, dried and concentrated the organic phase, mixed with silica gel and passed through the column ...
Embodiment 2
[0119]
[0120] Compound (X-2) (1mmol), intermediate (M-1) (2mmol), potassium tert-butoxide (2mmol), palladium acetate (5mol%), tricyclohexylphosphine (10mol%), anhydrous toluene 50mL , reacted at 80°C for 24 hours, cooled to room temperature, separated and extracted three times with dichloromethane, combined the organic phases, dried and concentrated the organic phases, mixed with silica gel and passed through the column to obtain 0.33 g of compound (A-3).
[0121] 1H NMR (400MHz, Solvent: DMSO-d6) δppm: 8.53(2H), 8.36(8H), 7.64(2H), 7.44-7.38(8H), 7.33-7.24(22H).
[0122]
Embodiment 3
[0124]
[0125] Compound (X-3) (1mmol), intermediate (M-1) (2mmol), potassium tert-butoxide (2mmol), palladium acetate (5mol%), tricyclohexylphosphine (10mol%), anhydrous toluene 50mL , reacted at 80°C for 24 hours, cooled to room temperature, separated and extracted three times with dichloromethane, combined the organic phase, concentrated the organic phase after drying, mixed the sample with silica gel and passed through the column to obtain 0.43 g of compound (B-1).
[0126] 1H NMR (400MHz, Solvent: DMSO-d6) δppm: 8.37(8H), 7.54(2H), 7.41(8H), 7.33-7.24(22H), 7.14(2H), 7.04(2H).
[0127]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com